Phosphatidylglycerols (egg) (sodium salt)
Modify Date: 2024-01-14 10:44:03
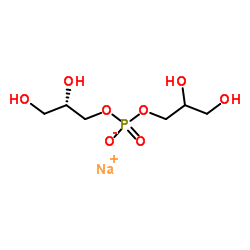
Phosphatidylglycerols (egg) (sodium salt) structure
|
Common Name | Phosphatidylglycerols (egg) (sodium salt) | ||
|---|---|---|---|---|
| CAS Number | 383907-64-0 | Molecular Weight | 268.134 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C6H14NaO8P | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Phosphatidylglycerols (egg) (sodium salt)Phosphatidylglycerol is a naturally occurring anionic phospholipid that is a component of plant, animal and bacterial cell membranes. It is present in prokaryotes and eukaryotes less than phosphatidylethanolamine, and in eukaryotes less than phosphatidylcholine. It is formed by the reaction between CDP-diglyceride and L-α-glycerol 3-phosphate followed by dephosphorylation and is the metabolic precursor of cardiolipin. Phosphatidylglycerols containing polyunsaturated and monounsaturated fatty acyl chains inhibit and promote the proliferation of murine keratinocytes, respectively. Phosphatidylglycerol is the second-largest lipid component of mammalian lung surfactant, accounting for 10% of lipids, and has reduced levels of pulmonary surfactant in infants with respiratory distress syndrome. Phosphatidylglycerol (egg) is a mixture of phosphatidylglycerols isolated from eggs with various fatty acyl groups at the sn-1 and sn-2 positions. References: [1]. Ohtsuka, T., Nishijima, M., and Akamatsu, Y. Phosphatidylglycerol phosphate synthase-deficient somatic mutants with impaired phosphatidylglycerol and cardiolipin biosynthesis J. Biol. Chemical. 268(30), 22908-22913 (1993).[2]. Furse, S. Are phosphatidylglycerols essential for terrestrial life? J. Chemistry. biology. 10(1), 1-9 (2016).[3]. Xie, D., Seremwe, M., Edwards, JG, et al. Different effects of different phosphatidylglycerols on the proliferation of mouse keratinocytes PLoS One 9(9), e107119 (2014). |
| Name | Sodium (2S)-2,3-dihydroxypropyl 2,3-dihydroxypropyl phosphate |
|---|---|
| Synonym | More Synonyms |
| Description | Phosphatidylglycerol is a naturally occurring anionic phospholipid that is a component of plant, animal and bacterial cell membranes. It is present in prokaryotes and eukaryotes less than phosphatidylethanolamine, and in eukaryotes less than phosphatidylcholine. It is formed by the reaction between CDP-diglyceride and L-α-glycerol 3-phosphate followed by dephosphorylation and is the metabolic precursor of cardiolipin. Phosphatidylglycerols containing polyunsaturated and monounsaturated fatty acyl chains inhibit and promote the proliferation of murine keratinocytes, respectively. Phosphatidylglycerol is the second-largest lipid component of mammalian lung surfactant, accounting for 10% of lipids, and has reduced levels of pulmonary surfactant in infants with respiratory distress syndrome. Phosphatidylglycerol (egg) is a mixture of phosphatidylglycerols isolated from eggs with various fatty acyl groups at the sn-1 and sn-2 positions. References: [1]. Ohtsuka, T., Nishijima, M., and Akamatsu, Y. Phosphatidylglycerol phosphate synthase-deficient somatic mutants with impaired phosphatidylglycerol and cardiolipin biosynthesis J. Biol. Chemical. 268(30), 22908-22913 (1993).[2]. Furse, S. Are phosphatidylglycerols essential for terrestrial life? J. Chemistry. biology. 10(1), 1-9 (2016).[3]. Xie, D., Seremwe, M., Edwards, JG, et al. Different effects of different phosphatidylglycerols on the proliferation of mouse keratinocytes PLoS One 9(9), e107119 (2014). |
|---|---|
| Related Catalog |
| Molecular Formula | C6H14NaO8P |
|---|---|
| Molecular Weight | 268.134 |
| Exact Mass | 268.032410 |
| Storage condition | -20°C |
| Hazard Class | 6.1 |
|---|
| Sodium (2S)-2,3-dihydroxypropyl 2,3-dihydroxypropyl phosphate |