Nalidixic acid

Nalidixic acid structure
|
Common Name | Nalidixic acid | ||
|---|---|---|---|---|
| CAS Number | 389-08-2 | Molecular Weight | 232.235 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 413.1±45.0 °C at 760 mmHg | |
| Molecular Formula | C12H12N2O3 | Melting Point | 227-229 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 203.6±28.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Nalidixic acidNalidixic acid is a synthetic 1,8-naphthyridine antimicrobial agent with a limited bacteriocidal spectrum.Target: AntibacterialNalidixic acid is the first of the synthetic quinolone antibiotics. Nalidixic acid is effective against both gram-positive and gram-negative bacteria. In lower concentrations, it acts in a bacteriostatic manner; that is, it inhibits growth and reproduction. In higher concentrations, it is bactericidal, meaning that it kills bacteria instead of merely inhibiting their growth. Nalidixic selectively and reversibly blocks DNA replication in susceptible bacteria. Nalidixic acid and related antibiotics inhibit a subunit of DNA gyrase and topoisomerase IV and induce formation of cleavage complexes. It also inhibits the nicking-closing activity on the subunit of DNA gyrase that releases the positive binding stress on the supercoiled DNA. From Wikipedia. |
| Name | nalidixic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Nalidixic acid is a synthetic 1,8-naphthyridine antimicrobial agent with a limited bacteriocidal spectrum.Target: AntibacterialNalidixic acid is the first of the synthetic quinolone antibiotics. Nalidixic acid is effective against both gram-positive and gram-negative bacteria. In lower concentrations, it acts in a bacteriostatic manner; that is, it inhibits growth and reproduction. In higher concentrations, it is bactericidal, meaning that it kills bacteria instead of merely inhibiting their growth. Nalidixic selectively and reversibly blocks DNA replication in susceptible bacteria. Nalidixic acid and related antibiotics inhibit a subunit of DNA gyrase and topoisomerase IV and induce formation of cleavage complexes. It also inhibits the nicking-closing activity on the subunit of DNA gyrase that releases the positive binding stress on the supercoiled DNA. From Wikipedia. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 413.1±45.0 °C at 760 mmHg |
| Melting Point | 227-229 °C(lit.) |
| Molecular Formula | C12H12N2O3 |
| Molecular Weight | 232.235 |
| Flash Point | 203.6±28.7 °C |
| Exact Mass | 232.084793 |
| PSA | 72.19000 |
| LogP | 1.19 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.605 |
| InChIKey | MHWLWQUZZRMNGJ-UHFFFAOYSA-N |
| SMILES | CCn1cc(C(=O)O)c(=O)c2ccc(C)nc21 |
| Storage condition | 0-6°C |
| Stability | Stable. Incompatible with strong oxidizing agents. |
| Water Solubility | 0.1 G/L (23 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R40;R42/43;R63 |
| Safety Phrases | S22-S36/37-S45-S24 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | QN2885000 |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Brucella abortus depends on pyruvate phosphate dikinase and malic enzyme but not on Fbp and GlpX fructose-1,6-bisphosphatases for full virulence in laboratory models.
J. Bacteriol. 196(16) , 3045-57, (2014) The brucellae are the etiological agents of brucellosis, a worldwide-distributed zoonosis. These bacteria are facultative intracellular parasites and thus are able to adjust their metabolism to the ex... |
|
|
Recovery of Pseudomonas aeruginosa culturability following copper- and chlorine-induced stress.
FEMS Microbiol. Lett. 356(2) , 226-34, (2014) This study investigated how quickly cells of the opportunistic pathogen Pseudomonas aeruginosa recover culturability after exposure to two of the most common environmental stressors present in drinkin... |
|
|
Intestinal Epithelial Cell Tyrosine Kinase 2 Transduces IL-22 Signals To Protect from Acute Colitis.
J. Immunol. 195 , 5011-24, (2015) In the intestinal tract, IL-22 activates STAT3 to promote intestinal epithelial cell (IEC) homeostasis and tissue healing. The mechanism has remained obscure, but we demonstrate that IL-22 acts via ty... |
| uriben |
| EINECS 206-864-7 |
| 1,8-Naphthyridine-3-carboxylic acid, 1-ethyl-1,4-dihydro-7-methyl-4-oxo- |
| uroman |
| uropan |
| NegGram |
| NOGRAM |
| WIN-18320 |
| nalidixic acid |
| Urisal |
| nalix |
| 1-Ethyl-7-methyl-4-oxo-1,4-dihydro-1,8-naphthyridine-3-carboxylic acid |
| Ethyl-7-methyl-1,8-naphthyridin-4-one-3-carboxylic Acid |
| 1-Ethyl-1,4-dihydro-7-methyl-4-oxo-1,8-naphthyridine-3-carboxylic Acid |
| T66 BV EN GNJ CVQ E2 H1 |
| poleon |
| negram |
| WINTOMYLON |
| cybis |
| uroneg |
| MFCD00006884 |
![[N-Ethyl-N-(6-methyl-2-pyridyl)amino]methylenemalonic acid diethyl ester Structure](https://image.chemsrc.com/caspic/223/34748-19-1.png) CAS#:34748-19-1
CAS#:34748-19-1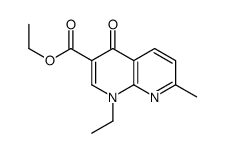 CAS#:33331-59-8
CAS#:33331-59-8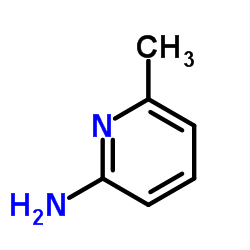 CAS#:1824-81-3
CAS#:1824-81-3![Propanedioic acid,2-[[(6-methyl-2-pyridinyl)amino]methylene]-, 1,3-diethyl ester Structure](https://image.chemsrc.com/caspic/281/13250-95-8.png) CAS#:13250-95-8
CAS#:13250-95-8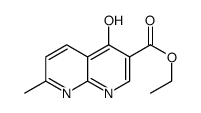 CAS#:13250-96-9
CAS#:13250-96-9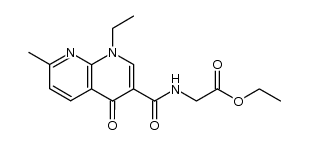 CAS#:174814-96-1
CAS#:174814-96-1 CAS#:10299-49-7
CAS#:10299-49-7
