3-tert-Butoxy-3-oxopropanoic acid
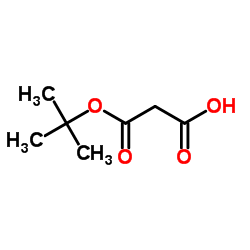
3-tert-Butoxy-3-oxopropanoic acid structure
|
Common Name | 3-tert-Butoxy-3-oxopropanoic acid | ||
|---|---|---|---|---|
| CAS Number | 40052-13-9 | Molecular Weight | 160.168 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 250.5±23.0 °C at 760 mmHg | |
| Molecular Formula | C7H12O4 | Melting Point | 19-20 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 97.7±16.1 °C | |
| Symbol |


GHS05, GHS06 |
Signal Word | Danger | |
| Name | 3-tert-Butoxy-3-oxopropanoic acid |
|---|---|
| Synonym | More Synonyms |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 250.5±23.0 °C at 760 mmHg |
| Melting Point | 19-20 °C(lit.) |
| Molecular Formula | C7H12O4 |
| Molecular Weight | 160.168 |
| Flash Point | 97.7±16.1 °C |
| Exact Mass | 160.073563 |
| PSA | 63.60000 |
| LogP | 1.04 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.444 |
| InChIKey | NGGGZUAEOKRHMA-UHFFFAOYSA-N |
| SMILES | CC(C)(C)OC(=O)CC(=O)O |
| Symbol |


GHS05, GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H314-H330 |
| Precautionary Statements | P260-P280-P284-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Faceshields;full-face respirator (US);Gloves;Goggles;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | T |
| Risk Phrases | R26 |
| Safety Phrases | S26-S27-S36/37/39-S45 |
| RIDADR | UN 2922 8/PG 3 |
| WGK Germany | 3 |
| Hazard Class | 8.0, 6.1 |
| HS Code | 2918990090 |
| Precursor 10 | |
|---|---|
| DownStream 9 | |
| HS Code | 2918990090 |
|---|---|
| Summary | 2918990090. other carboxylic acids with additional oxygen function and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Development of an enzyme-linked immunosorbent assay for the detection of the organophosphorus insecticide acephate.
J. Agric. Food Chem. 51(13) , 3695-703, (2003) A competitive indirect enzyme-linked immunosorbent assay (ciELISA) for the organophosphorus insecticide acephate, O,S-dimethyl acetylphosphoramidothioate, was developed using a polyclonal antibody. Fi... |
|
|
A thermostable L-aminoacylase from Thermococcus litoralis: cloning, overexpression, characterization, and applications in biotransformations.
Extremophiles 6(2) , 111-22, (2002) A thermostable L-aminoacylase from Thermococcus litoralis was cloned, sequenced, and overexpressed in Escherichia coli. The enzyme is a homotetramer of 43 kDa monomers and has an 82% sequence identity... |
|
|
Formation of high-aspect-ratio helical nanorods via chiral self-assembly of fullerodendrimers. Hilmer AJ, et al.
J. Phys. Chem. Lett. 5(5) , 929-34, (2014)
|
| 3-[(2-Methyl-2-propanyl)oxy]-3-oxopropanoic acid |
| mono-tert-Butyl malonate |
| 3-[(2-methylpropan-2-yl)oxy]-3-oxopropanoic acid |
| Propanedioic acid, mono(1,1-dimethylethyl) ester |
| tert-Butyl Hydrogen Malonate |
| Malonic Acid Mono-tert-butyl Ester |
| MFCD00191886 |
| 3-tert-Butoxy-3-oxopropanoic acid |
| 3-(tert-Butoxy)-3-oxopropanoic acid |
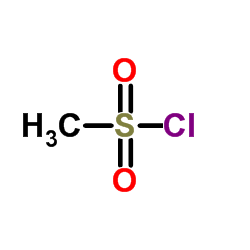 CAS#:124-63-0
CAS#:124-63-0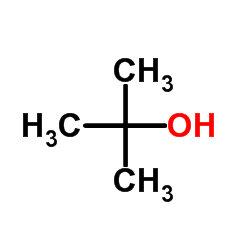 CAS#:75-65-0
CAS#:75-65-0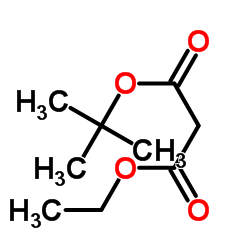 CAS#:32864-38-3
CAS#:32864-38-3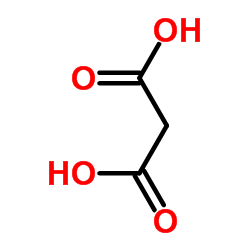 CAS#:141-82-2
CAS#:141-82-2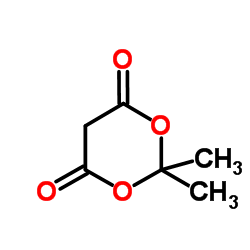 CAS#:2033-24-1
CAS#:2033-24-1 CAS#:24424-99-5
CAS#:24424-99-5 CAS#:540-88-5
CAS#:540-88-5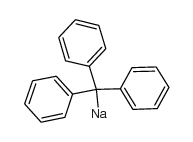 CAS#:4303-71-3
CAS#:4303-71-3 CAS#:42726-73-8
CAS#:42726-73-8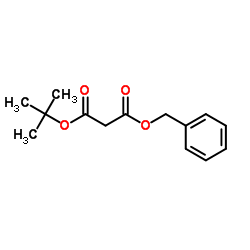 CAS#:72594-86-6
CAS#:72594-86-6 CAS#:124-38-9
CAS#:124-38-9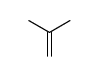 CAS#:115-11-7
CAS#:115-11-7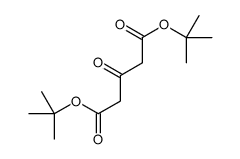 CAS#:28009-80-5
CAS#:28009-80-5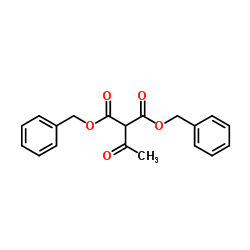 CAS#:28009-82-7
CAS#:28009-82-7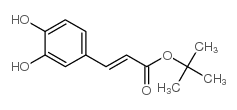 CAS#:845883-04-7
CAS#:845883-04-7 CAS#:42998-54-9
CAS#:42998-54-9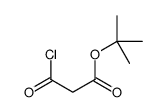 CAS#:53075-12-0
CAS#:53075-12-0
