(3-beta,5-beta,6-beta)-5,6-Epoxycholestan-3-ol
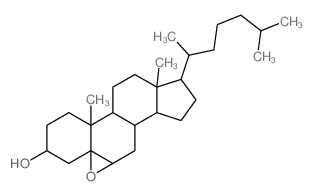
(3-beta,5-beta,6-beta)-5,6-Epoxycholestan-3-ol structure
|
Common Name | (3-beta,5-beta,6-beta)-5,6-Epoxycholestan-3-ol | ||
|---|---|---|---|---|
| CAS Number | 4025-59-6 | Molecular Weight | 402.65300 | |
| Density | 1.04g/cm3 | Boiling Point | 497.7ºC at 760 mmHg | |
| Molecular Formula | C27H46O2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 200.9ºC | |
Use of (3-beta,5-beta,6-beta)-5,6-Epoxycholestan-3-olCholesterol 5beta,6beta-epoxide is an oxidative metabolite of cholesterol formed by free-radical and non-radical oxidation of cholesterol at the 5,6 double bond. [1] [2] Induces lactate dehydrogenase (LDH) release and apoptosis in macrophage-differentiated U937 cells. [3] 5β,6β-epoxycholestanol has been found in human fatty streaks and advanced atherosclerotic lesions, but not in normal aortic tissue. [4] References: [1]. Pulfer, MK, and Murphy, RC Formation of bioactive oxygen sterols during ozonolysis of cholesterol present in lung surfactants. Journal of Biochemistry 279(25), 26331-26338 (2004). [2]. Aringer, L. and Eneroth, P. In vitro formation and metabolism of cholesterol and β-sitosterol 5,6-epoxides. J. Lipid Research. 15(4), 389-398 (1974).[3]. Lordan, S., O'Brien, NM, and Mackrill, JJ Calcium in 7β-hydroxycholesterol and cholesterol-5β,6β-epoxide-induced apoptosis. J. Biochemistry. Moore. poison. 23(5), 324-332 (2009).[4]. Garcia-Cruset, S., Carpenter, KL, Guardiola, F. et al. Oxysterol profiles of normal human arteries, fatty streaks, and advanced lesions. free radicals. reservoir. 35(1), 31-41 (2001). |
| Name | 5,6β-epoxy-5β-cholestan-3β-ol |
|---|---|
| Synonym | More Synonyms |
| Description | Cholesterol 5beta,6beta-epoxide is an oxidative metabolite of cholesterol formed by free-radical and non-radical oxidation of cholesterol at the 5,6 double bond. [1] [2] Induces lactate dehydrogenase (LDH) release and apoptosis in macrophage-differentiated U937 cells. [3] 5β,6β-epoxycholestanol has been found in human fatty streaks and advanced atherosclerotic lesions, but not in normal aortic tissue. [4] References: [1]. Pulfer, MK, and Murphy, RC Formation of bioactive oxygen sterols during ozonolysis of cholesterol present in lung surfactants. Journal of Biochemistry 279(25), 26331-26338 (2004). [2]. Aringer, L. and Eneroth, P. In vitro formation and metabolism of cholesterol and β-sitosterol 5,6-epoxides. J. Lipid Research. 15(4), 389-398 (1974).[3]. Lordan, S., O'Brien, NM, and Mackrill, JJ Calcium in 7β-hydroxycholesterol and cholesterol-5β,6β-epoxide-induced apoptosis. J. Biochemistry. Moore. poison. 23(5), 324-332 (2009).[4]. Garcia-Cruset, S., Carpenter, KL, Guardiola, F. et al. Oxysterol profiles of normal human arteries, fatty streaks, and advanced lesions. free radicals. reservoir. 35(1), 31-41 (2001). |
|---|---|
| Related Catalog |
| Density | 1.04g/cm3 |
|---|---|
| Boiling Point | 497.7ºC at 760 mmHg |
| Molecular Formula | C27H46O2 |
| Molecular Weight | 402.65300 |
| Flash Point | 200.9ºC |
| Exact Mass | 402.35000 |
| PSA | 32.76000 |
| LogP | 6.59990 |
| Vapour Pressure | 5.43E-12mmHg at 25°C |
| Index of Refraction | 1.533 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
|---|---|
| Hazard Codes | Xn |
| Risk Phrases | 20/21/22-48/20/21/22 |
| Safety Phrases | 7-36/37 |
| RIDADR | NONH for all modes of transport |
|
Surprising unreactivity of cholesterol-5,6-epoxides towards nucleophiles.
J. Lipid Res. 53(4) , 718-25, (2012) We recently established that drugs used for the treatment and the prophylaxis of breast cancers, such as tamoxifen, were potent inhibitors of cholesterol-5,6-epoxide hydrolase (ChEH), which led to the... |
|
|
Formation of biologically active oxysterols during ozonolysis of cholesterol present in lung surfactant.
J. Biol. Chem. 279(25) , 26331-8, (2004) Exposure of the lung to concentrations of ozone found in ambient air is known to cause toxicity to the epithelial cells of the lung. Because of the chemical reactivity of ozone, it likely reacts with ... |
|
|
Comparative study of the cytotoxicity and apoptosis-inducing potential of commonly occurring oxysterols.
Cell Biol. Toxicol. 17(2) , 127-37, (2001) The cytotoxicity of the oxysterols 25-hydroxycholesterol, 7beta-hydroxycholesterol, cholesterol-5alpha,6alphaepoxide, cholesterol-5beta,6beta-epoxide, 19-hydroxycholesterol and 7-ketocholesterol was e... |
| 5beta,6beta-Epoxycholestanol |
| CHOLESTEROL-5BETA,6BETA-EPOXIDE |
| 5,6beta-epoxy-5beta-cholestan-3beta-ol |
| 5BETA,6BETA-EPOXYCHOLESTAN-3BETA-OL |
| cholestanol,5ß,6ß-epo |
| cholesterol-5B,6B-epoxide |
| cholesterolbeta-epoxide |