Framycetin sulfate
Modify Date: 2025-08-27 10:07:00
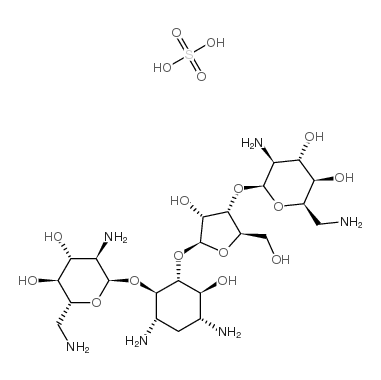
Framycetin sulfate structure
|
Common Name | Framycetin sulfate | ||
|---|---|---|---|---|
| CAS Number | 4146-30-9 | Molecular Weight | 712.72200 | |
| Density | N/A | Boiling Point | 927.1ºC at 760 mmHg | |
| Molecular Formula | C23H46N6O13.H2SO4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 514.5ºC | |
Use of Framycetin sulfateFramycetin sulfate (Neomycin B sulfate), an aminoglycoside antibiotic, is a potent RNase P cleavage activity inhibitor with a Ki of 35 μM. Framycetin sulfate competes for specific divalent metal ion binding sites in RNase P RNA. Framycetin sulfate inhibits hammerhead ribozyme with a Ki of 13.5 μM. Framycetin sulfate, a 5″-azido neomycin B precursor, binds the Drosha site in miR-525 and is used for hepatic encephalopathy and enteropathogenic E. coli infections[1][2]. |
| Name | (2R,3S,4R,5R,6R)-5-amino-2-(aminomethyl)-6-[(1R,2R,3S,4R,6S)-4,6-diamino-2-[(2S,3R,4S,5R)-4-[(2S,3S,4S,5R,6R)-3-amino-6-(aminomethyl)-4,5-dihydroxyoxan-2-yl]oxy-3-hydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy-3-hydroxycyclohexyl]oxyoxane-3,4-diol,sulfuric acid |
|---|---|
| Synonym | More Synonyms |
| Description | Framycetin sulfate (Neomycin B sulfate), an aminoglycoside antibiotic, is a potent RNase P cleavage activity inhibitor with a Ki of 35 μM. Framycetin sulfate competes for specific divalent metal ion binding sites in RNase P RNA. Framycetin sulfate inhibits hammerhead ribozyme with a Ki of 13.5 μM. Framycetin sulfate, a 5″-azido neomycin B precursor, binds the Drosha site in miR-525 and is used for hepatic encephalopathy and enteropathogenic E. coli infections[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 35 μM (RNase P cleavage activity) and 13.5 μM (hammerhead ribozyme)[1] |
| In Vitro | The inhibition of RNase P RNA cleavage by Framycetin sulfate (Neomycin Bsulfate; Fradiomycin Bsulfate) is sensitive to pH and an increase in pH suppresses the inhibition in other systems[1]. Framycetin sulfate targets the bacterial and human ribosome and affect translation. 5″-azido neomycin B and Framycetin sulfate selectively inhibit production of the mature miRNA, boosts a downstream protein, and inhibits invasion in HCC cell line[2]. Framycetin sulfate binds to a structural rather than a sequence motif of the RNA. Its primary cognate target is the decoding site of the 16S rRNA, but it also binds to the Rev-responsive element in HIV-1, group I introns, and the hammerhead ribozyme, and thus inhibits their biological function[3]. Framycetin sulfate induces misreading of the genetic code during translation and inhibits several ribozymes. The ribosomal target site is the 16 S rRNA 1400 to 1500 region[4]. |
| References |
| Boiling Point | 927.1ºC at 760 mmHg |
|---|---|
| Molecular Formula | C23H46N6O13.H2SO4 |
| Molecular Weight | 712.72200 |
| Flash Point | 514.5ºC |
| Exact Mass | 712.28000 |
| PSA | 436.09000 |
| InChIKey | OIXVKQDWLFHVGR-WQDIDPJDSA-N |
| SMILES | NCC1OC(OC2C(CO)OC(OC3C(O)C(N)CC(N)C3OC3OC(CN)C(O)C(O)C3N)C2O)C(N)C(O)C1O.O=S(=O)(O)O |
| Hazard Codes | Xi |
|---|
| Precursor 0 | |
|---|---|
| DownStream 2 | |
| Neomycin B trisulfate |
| neomycin sulphate |
| Neohalicholactone |
| neomycin B sulfate |
| EINECS 223-969-3 |
| Framycetin sulphate |
| neomycin trisulfate |
 CAS#:2037-48-1
CAS#:2037-48-1 CAS#:84107-26-6
CAS#:84107-26-6