1,1,1-Trifluoroacetone

1,1,1-Trifluoroacetone structure
|
Common Name | 1,1,1-Trifluoroacetone | ||
|---|---|---|---|---|
| CAS Number | 421-50-1 | Molecular Weight | 112.050 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 21.5±0.0 °C at 760 mmHg | |
| Molecular Formula | C3H3F3O | Melting Point | -78 °C | |
| MSDS | Chinese USA | Flash Point | -30.6±0.0 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Danger | |
| Name | 1,1,1-Trifluoroacetone |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 21.5±0.0 °C at 760 mmHg |
| Melting Point | -78 °C |
| Molecular Formula | C3H3F3O |
| Molecular Weight | 112.050 |
| Flash Point | -30.6±0.0 °C |
| Exact Mass | 112.013596 |
| PSA | 17.07000 |
| LogP | 0.49 |
| Vapour Pressure | 865.9±0.0 mmHg at 25°C |
| Index of Refraction | 1.287 |
| InChIKey | FHUDAMLDXFJHJE-UHFFFAOYSA-N |
| SMILES | CC(=O)C(F)(F)F |
| Storage condition | 2-8°C |
| Water Solubility | Miscible |
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H224-H315-H319-H335 |
| Precautionary Statements | P210-P261-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US) |
| Hazard Codes | F+:Highlyflammable;Xi:Irritant; |
| Risk Phrases | R12;R36/37/38 |
| Safety Phrases | S16-S26-S29-S33-S36-S7/9-S9-S37/39 |
| RIDADR | UN 1993 3/PG 1 |
| WGK Germany | 3 |
| Packaging Group | I |
| Hazard Class | 3 |
| HS Code | 29147090 |
| Precursor 9 | |
|---|---|
| DownStream 9 | |
| HS Code | 2914700090 |
|---|---|
| Summary | HS: 2914700090 halogenated, sulphonated, nitrated or nitrosated derivatives of ketones and quinones, whether or not with other oxygen function Tax rebate rate:9.0% Supervision conditions:none VAT:17.0% MFN tariff:5.5% General tariff:30.0% |
|
Mitochondrial dysfunction and respiratory chain defects in a rodent model of methotrexate-induced enteritis.
Hum. Exp. Toxicol. 33(10) , 1051-65, (2014) The efficacy of methotrexate (MTX), a widely used chemotherapeutic drug, is limited by its gastrointestinal toxicity and the mechanism of which is not clear. The present study investigates the possibl... |
|
|
Concise synthesis of enantiopure alpha-trifluoromethyl alanines, diamines, and amino alcohols via the Strecker-type reaction.
J. Org. Chem. 71 , 7075, (2006) Diastereomerically pure alpha-trifluoromethyl alpha-amino nitriles obtained by Strecker-type reactions from chiral CF3 imines and iminium proved to be very attractive versatile intermediates for the s... |
|
|
Synthesis , 251, (2007)
|
| 1,1,1-Trifluorpropan-2-on |
| EINECS 207-005-9 |
| 1,1,1-Trifluoroacetone |
| 2-Propanone, 1,1,1-trifluoro- |
| MFCD00000423 |
| 1,1,1-trifluoropropan-2-one |
 CAS#:431-37-8
CAS#:431-37-8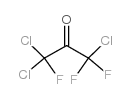 CAS#:79-52-7
CAS#:79-52-7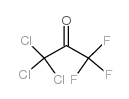 CAS#:758-42-9
CAS#:758-42-9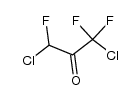 CAS#:56753-83-4
CAS#:56753-83-4 CAS#:126266-75-9
CAS#:126266-75-9 CAS#:64-19-7
CAS#:64-19-7 CAS#:76-05-1
CAS#:76-05-1 CAS#:108-24-7
CAS#:108-24-7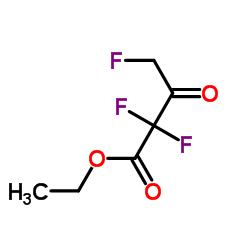 CAS#:372-31-6
CAS#:372-31-6 CAS#:338-03-4
CAS#:338-03-4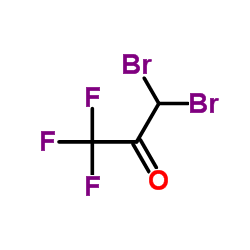 CAS#:431-67-4
CAS#:431-67-4 CAS#:507-52-8
CAS#:507-52-8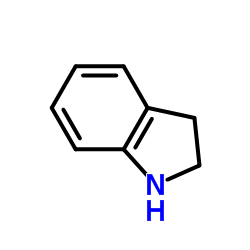 CAS#:496-15-1
CAS#:496-15-1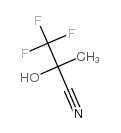 CAS#:335-08-0
CAS#:335-08-0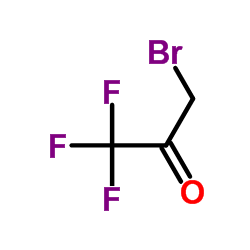 CAS#:431-35-6
CAS#:431-35-6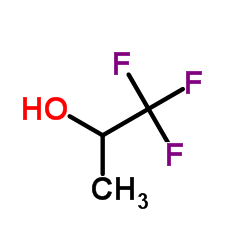 CAS#:374-01-6
CAS#:374-01-6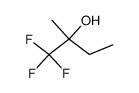 CAS#:507-54-0
CAS#:507-54-0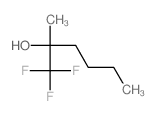 CAS#:14633-64-8
CAS#:14633-64-8