1-Dodecylimidazole
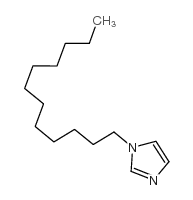
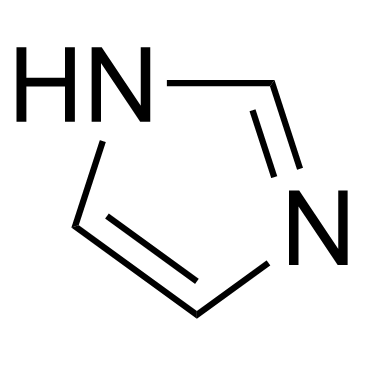
1-Dodecylimidazole structure
|
Common Name | 1-Dodecylimidazole | ||
|---|---|---|---|---|
| CAS Number | 4303-67-7 | Molecular Weight | 236.39600 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C15H28N2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of 1-Dodecylimidazole1-Dodecylimidazole (N-Dodecylimidazole) is a lysosomotropic detergent and a cytotoxic agent. 1-Dodecylimidazole causes cell death by its acid-dependent accumulation in lysosomes, disruption of the lysosomal membrane, and releaseof cysteine proteases into the cytoplasm. 1-Dodecylimidazole has hypocholesterolaemic activity and broad-spectrum antifungal activity[1][2][3]. |
| Name | 1-dodecylimidazole |
|---|---|
| Synonym | More Synonyms |
| Description | 1-Dodecylimidazole (N-Dodecylimidazole) is a lysosomotropic detergent and a cytotoxic agent. 1-Dodecylimidazole causes cell death by its acid-dependent accumulation in lysosomes, disruption of the lysosomal membrane, and releaseof cysteine proteases into the cytoplasm. 1-Dodecylimidazole has hypocholesterolaemic activity and broad-spectrum antifungal activity[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | N-dodecylimidazole, an acid activated detergent with a pKa of 6.3, has been shown to be cytotoxic to cells in culture. N-dodecylimidazole displayed extracellular pH (pHe)-dependent cytotoxicity against EMT-6 and MGH U1 cells. cell killing was dose dependent and was 100-fold greater at pHe 6.0 than pHe 7.0[4]. |
| In Vivo | The hypocholesterolaemic activity of 1-dodecylimidazole results in part from the inhibition of cholesterol biosynthesis at the level of 2,3-oxidosqualene sterol cyclase[2]. 1-dodecylimidazole (150 mg/kg body wt; by stomach tube; daily for 10 days) has lower serum cholesterol concentrations than control rats[2]. Animal Model: Male rats[2] Dosage: 150 mg/kg body wt Administration: By stomach tube; daily for 10 days Result: Had significantly lower serum cholesterol concentrations than untreated animals. |
| References |
| Molecular Formula | C15H28N2 |
|---|---|
| Molecular Weight | 236.39600 |
| Exact Mass | 236.22500 |
| PSA | 17.82000 |
| LogP | 4.80400 |
| InChIKey | JMTFLSQHQSFNTE-UHFFFAOYSA-N |
| SMILES | CCCCCCCCCCCCn1ccnc1 |
| Storage condition | 2~8℃,Seal |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
|
~92% 
1-Dodecylimidazole CAS#:4303-67-7 |
| Literature: Kapitanov; Belousova; Shumeiko; Kostrikin; Prokop'Eva; Popov Russian Journal of Organic Chemistry, 2013 , vol. 49, # 9 p. 1291 - 1299 Zh. Org. Khim., 2013 , vol. 49, # 9 p. 1308 - 1316,9 |
|
~% 
1-Dodecylimidazole CAS#:4303-67-7 |
| Literature: Abate, Antonio; Petrozza, Annamaria; Cavallo, Gabriella; Lanzani, Guglielmo; Matteucci, Francesco; Bruce, Duncan W.; Houbenov, Nikolay; Metrangolo, Pierangelo; Resnati, Giuseppe Journal of Materials Chemistry A, 2013 , vol. 1, # 22 p. 6572 - 6578 |
|
~96% 
1-Dodecylimidazole CAS#:4303-67-7 |
| Literature: Qiao, Yunxiang; Hou, Zhenshan; Li, Huan; Hu, Yu; Feng, Bo; Wang, Xiangrui; Hua, Li; Huang, Qingfa Green Chemistry, 2009 , vol. 11, # 12 p. 1955 - 1960 |
|
~83% 
1-Dodecylimidazole CAS#:4303-67-7 |
| Literature: Savignac, A. de; Roques, C.; Hinedi, M.; Michel, G.; Lattes, A. European Journal of Medicinal Chemistry, 1990 , vol. 25, # 5 p. 449 - 454 |
|
~% 
1-Dodecylimidazole CAS#:4303-67-7 |
| Literature: BASF Aktiengesellschaft Patent: US4450277 A1, 1984 ; |
|
~58% 
1-Dodecylimidazole CAS#:4303-67-7 |
| Literature: Martinez, Regina; Torregrosa, Rosario; Pastor, Isidro M.; Yus, Miguel Synthesis (Germany), 2012 , vol. 44, # 16 p. 2630 - 2638 |
|
~% 
1-Dodecylimidazole CAS#:4303-67-7 |
| Literature: Liu, Xue-Feng; Dong, Li-Li; Fang, Yun Journal of Surfactants and Detergents, 2011 , vol. 14, # 4 p. 497 - 504 |
|
~26% 
1-Dodecylimidazole CAS#:4303-67-7 |
| Literature: Abramzon; Pevzner; Kofman; Alam Russian Journal of Applied Chemistry, 1996 , vol. 69, # 12 p. 1841 - 1848 |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| Imidazole,1-dodecyl |
| 1-n-dodecanylimidazole |
| 1H-Imidazole,1-dodecyl |
| dodecylimidazole |
| EINECS 224-314-4 |
| N-dodecyl imidazole |
| 1-Laurylimidazole |
| 1-Dodecylimidazole |
| 1-Dodecyl-1H-imidazole |
| N-Laurylimidazole |