| Description |
Marizomib (Salinosporamide A) is second-generation, irreversible, brain-penetrant, pan-proteasome inhibitor. Marizomib inhibits the CT-L (β5), CT-T-laspase-like (C-L, β1) and trypsin-like (T-L, β2) activities of the 20S proteasome (IC50=3.5, 28, and 430 nM, respectively)[1][2][3].
|
| Related Catalog |
|
| Target |
IC50: 3.5 nM (CT-L), 28 nM (CT-T-laspase-like), 430 nM (trypsin-like)[1]
|
| In Vitro |
Marizomib (Salinosporamide A) (0.1-10000 nM; 72 hours) effectively reduces survival of D-54 and U-251 cells in a dose-dependent manner. The IC50s are ∼52 nM for U-251 and ∼20 nM for D-54[1]. Marizomib (24 hours; 60 nM) induces apoptosis and caspase-3 activation in glioma cells[1]. Cell Proliferation Assay[1] Cell Line: U-251 and D-54 cells Concentration: 0.1, 1, 10, 100, 1000, 10000 nM Incubation Time: 72 hours Result: Effectively reduced survival of D-54 and U-251 cells in a dose-dependent manner. Apoptosis Analysis[1] Cell Line: D-54 cells Concentration: 60 nM Incubation Time: 24 hours Result: Induces D-54 cells apoptosis. Western Blot Analysis[1] Cell Line: D-54 cells Concentration: 60 nM Incubation Time: 24 hours Result: Led to increased activity of caspase-3 in a dose-dependent manner.
|
| In Vivo |
Marizomib (Salinosporamide A) (0.15 mg/kg; i.v; twice a week for three weeks) significantly decreases tumor growth, and is not associated with any toxicity[3]. Animal Model: CB-17 SCID-male mice (4-6 weeks old)[3] Dosage: 0.15 mg/kg Administration: i.v; twice a week for three weeks Result: Significantly decreased tumor growth, and was not associated with any toxicity.
|
| References |
[1]. Di K, et al. Marizomib activity as a single agent in malignant gliomas: ability to cross the blood-brainbarrier. Neuro Oncol. 2016 Jun;18(6):840-8. [2]. Kale AJ, et al. Molecular mechanisms of acquired proteasome inhibitor resistance. J Med Chem. 2012 Dec 13;55(23):10317-27. [3]. Singh AV, et al. Pharmacodynamic and efficacy studies of the novel proteasome inhibitor NPI-0052 (marizomib) in a human plasmacytoma xenograft murine model. Br J Haematol. 2010 May;149(4):550-9.
|
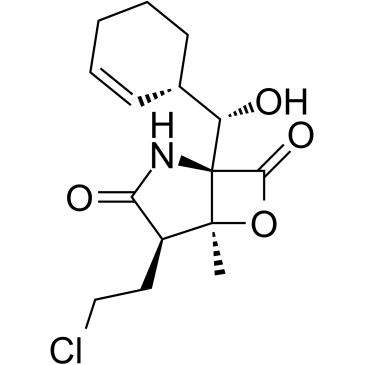
![(1R,4R,5S)-1-((S)-((S)-cyclohex-2-enyl)hydroxymethyl)-4-(2-hydroxyethyl)-5-methyl-6-oxa-2-azabicyclo[3.2.0]heptane-3,7-dione Structure](https://image.chemsrc.com/caspic/464/823229-54-5.png) CAS#:823229-54-5
CAS#:823229-54-5methyl}-1-methyl-7-oxa-4-aza-bicyclo[3.2.0]heptane-3,6-dione Structure](https://image.chemsrc.com/caspic/484/1064062-25-4.png) CAS#:1064062-25-4
CAS#:1064062-25-4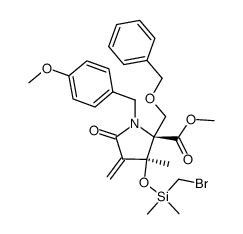 CAS#:704910-36-1
CAS#:704910-36-1