(-)-Borneol

(-)-Borneol structure
|
Common Name | (-)-Borneol | ||
|---|---|---|---|---|
| CAS Number | 464-45-9 | Molecular Weight | 154.249 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 212.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C10H18O | Melting Point | 206-209 °C | |
| MSDS | Chinese USA | Flash Point | 65.6±0.0 °C | |
| Symbol |


GHS02, GHS07 |
Signal Word | Warning | |
Use of (-)-Borneol(-)-Borneol has a highly efficacious positive modulating action at GABA receptor with an EC50 of 237 μM. |
| Name | (-)-borneol |
|---|---|
| Synonym | More Synonyms |
| Description | (-)-Borneol has a highly efficacious positive modulating action at GABA receptor with an EC50 of 237 μM. |
|---|---|
| Related Catalog | |
| Target |
EC50: 237 μM (GABA receptor)[1] |
| In Vitro | (-)-Borneol is an enantiomer of (+)-Borneol. (+)-Borneol is a natural bicyclic monoterpene used for analgesia and anesthesia in traditional Chinese medicine; enhances GABA receptor activity with an EC50 of 248 μM. At high concentrations (>1.5 mM), (-)-Borneol directly activates GABAA receptors producing 84% of the maximal GABA response indicative of a weak partial agonist action. (-)-Borneol produces dose-dependent positive modulation of the Cl- conductance generated by extremely low dose GABA at α1β2γ2L GABAA receptors[1]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 212.0±0.0 °C at 760 mmHg |
| Melting Point | 206-209 °C |
| Molecular Formula | C10H18O |
| Molecular Weight | 154.249 |
| Flash Point | 65.6±0.0 °C |
| Exact Mass | 154.135757 |
| PSA | 20.23000 |
| LogP | 2.71 |
| Vapour density | 5.31 (vs air) |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.502 |
| Water Solubility | INSOLUBLE |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H228-H317 |
| Precautionary Statements | P210-P280 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | F:Flammable; |
| Risk Phrases | R11 |
| Safety Phrases | S16-S36/37 |
| RIDADR | UN 1312 4.1/PG 3 |
| WGK Germany | 2 |
| RTECS | DT5095000 |
| Packaging Group | III |
| Hazard Class | 4.1 |
| HS Code | 29061900 |
| Precursor 5 | |
|---|---|
| DownStream 10 | |
| HS Code | 2906199090 |
|---|---|
| Summary | 2906199090. cyclanic, cyclenic or cyclotherpenic alcohols. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
Kinetics and mechanisms of the tropospheric reactions of menthol, borneol, fenchol, camphor, and fenchone with hydroxyl radicals (OH) and chlorine atoms (Cl).
J. Phys. Chem. A 116(16) , 4097-107, (2012) Relative kinetic techniques have been used to measure the rate coefficients for the reactions of oxygenated terpenes (menthol, borneol, fenchol, camphor, and fenchone) and cyclohexanol with hydroxyl r... |
|
|
Treatment with cardiotonic pills(®) after ischemia-reperfusion ameliorates myocardial fibrosis in rats.
Microcirculation 20(1) , 17-29, (2013) The present study was designed to evaluate whether CP was beneficial in alleviating myocardial fibrosis following I/R injury.Sprague-Dawley rats were subjected to 30 minutes occlusion of the LADCA, fo... |
|
|
Borneol alleviates oxidative stress via upregulation of Nrf2 and Bcl-2 in SH-SY5Y cells.
Pharm. Biol. 51(1) , 30-5, (2013) The β-amyloid (Aβ) peptide aggregation with accompanying oxidative stress plays the major role in the pathogenesis of Alzheimer's disease (AD). Some natural compounds, including borneol, shed promisin... |
| L-BORNEOL |
| l-2-Bornanol |
| Borneol |
| endo-(1S)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ol |
| endo-2-hydroxycamphane |
| 2-Camphanol |
| L(-)-Borneol |
| (1S,2R,4S)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ol |
| [(1s)-endo]-(-)-borneol |
| EINECS 207-353-1 |
| (1S-endo)-1,7,7-Trimethylbicyclo(2.2.1)heptan-2-ol |
| (-)-Borneol |
| (−)-Borneol |
| FEMA 2157 |
| (1S)-endo-(-)-Borneol |
| 2-bornanol |
| Bornan-2-ol |
| MFCD00003759 |
| (?)-Borneol |
| endo-2-Bornanol |
| Linderol |
| endo-1,7,7-trimethylbicyclo[2.2.1]heptan-2-ol |
| endo-2-camphanol |
| ngaicamphor |
| bornel |
![[2(R)-(1-bornyloxy)-5,6-dihydro-2H-pyran-5(R)-yl]acetic acid Structure](https://image.chemsrc.com/caspic/008/100703-54-6.png) CAS#:100703-54-6
CAS#:100703-54-6![(1S,2R,4S)-2-((4-methoxybenzyl)oxy)-1,7,7-trimethylbicyclo[2.2.1]heptane Structure](https://image.chemsrc.com/caspic/121/54384-77-9.png) CAS#:54384-77-9
CAS#:54384-77-9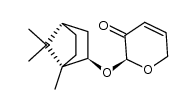 CAS#:100703-55-7
CAS#:100703-55-7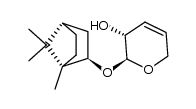 CAS#:100837-41-0
CAS#:100837-41-0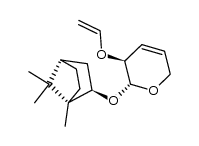 CAS#:100703-52-4
CAS#:100703-52-4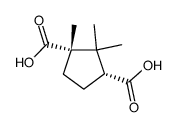 CAS#:560-09-8
CAS#:560-09-8 CAS#:79-92-5
CAS#:79-92-5![3-bromo-4,7,7-trimethylbicyclo[2.2.1]heptane structure](https://image.chemsrc.com/caspic/338/4443-48-5.png) CAS#:4443-48-5
CAS#:4443-48-5 CAS#:4501-58-0
CAS#:4501-58-0 CAS#:464-48-2
CAS#:464-48-2 CAS#:93-53-8
CAS#:93-53-8 CAS#:81115-69-7
CAS#:81115-69-7 CAS#:81115-66-4
CAS#:81115-66-4 CAS#:20279-31-6
CAS#:20279-31-6 CAS#:20279-25-8
CAS#:20279-25-8
