AUDA
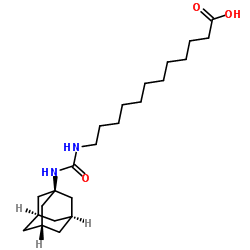
AUDA structure
|
Common Name | AUDA | ||
|---|---|---|---|---|
| CAS Number | 479413-70-2 | Molecular Weight | 392.575 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 592.7±19.0 °C at 760 mmHg | |
| Molecular Formula | C23H40N2O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 312.3±21.5 °C | |
Use of AUDAAUDA (compound 43) is a potent soluble epoxide hydrolase (sEH) inhibitor with IC50s of 18 and 69 nM for the mouse and human sEH, respectively[1]. AUDA has anti-inflammatory activity[2]. |
| Name | 12-(1-adamantylcarbamoylamino)dodecanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | AUDA (compound 43) is a potent soluble epoxide hydrolase (sEH) inhibitor with IC50s of 18 and 69 nM for the mouse and human sEH, respectively[1]. AUDA has anti-inflammatory activity[2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 18 nM (mouse sEH) and 69 nM (human sEH)[1] |
| In Vitro | AUDA (0.3-10 μg/mL; 48 hours) dose-dependently suppresses the proliferation of rat VSMCs exposed to PDGF[2]. AUDA (0.3-10 μg/mL; 30 min) dose-dependently upregulats COX-2 expression[2]. AUDA (10, 50 and 100 μM) augments the migratory ability of HCAECs in a dose-dependent manner[3]. AUDA significantly increases the adhesion ability of HCAECs[3]. Cell Proliferation Assay[2] Cell Line: Vascular smooth muscle cell (VSMC) Concentration: 0.3, 1, 3, 10 μg/mL Incubation Time: 48 hours Result: Dose-dependently suppressed the proliferation of rat VSMCs exposed to PDGF. Western Blot Analysis[2] Cell Line: VSMC Concentration: 1, 3, 10, 30 μg/mL Incubation Time: 30 min Result: Dose-dependently upregulated COX-2 expression. |
| In Vivo | AUDA (i.p.; 10 mg/kg; 14 days) reduces TNF-α, MMP-9 and IL-1β expression levels[3]. Animal Model: Male (wild-type) C57BL/6 mice (age, 4-6 weeks; weight, 18-20 g)[3] Dosage: 10 mg/kg Administration: i.p.; 14 days Result: Reduced TNF-α, MMP-9 and IL-1β expression levels. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 592.7±19.0 °C at 760 mmHg |
| Molecular Formula | C23H40N2O3 |
| Molecular Weight | 392.575 |
| Flash Point | 312.3±21.5 °C |
| Exact Mass | 392.303894 |
| PSA | 78.43000 |
| LogP | 5.61 |
| Vapour Pressure | 0.0±3.6 mmHg at 25°C |
| Index of Refraction | 1.534 |
| InChIKey | XLGSEOAVLVTJDH-UHFFFAOYSA-N |
| SMILES | O=C(O)CCCCCCCCCCCNC(=O)NC12CC3CC(CC(C3)C1)C2 |
| Storage condition | -20℃ |
| RIDADR | NONH for all modes of transport |
|---|
|
~98% 
AUDA CAS#:479413-70-2 |
| Literature: THE REGENTS OF THE UNIVERSITY OF CALIFORNIA Patent: WO2006/45119 A2, 2006 ; Location in patent: Page/Page column 44; 52 ; WO 2006/045119 A2 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Intimal smooth muscle cells are a source but not a sensor of anti-inflammatory CYP450 derived oxylipins.
Biochem. Biophys. Res. Commun. 463 , 774-80, (2015) Vascular pathologies are associated with changes in the presence and expression of morphologically distinct vascular smooth muscle cells. In particular, in complex human vascular lesions and models of... |
|
|
Alkylphloroglucinol derivatives and triterpenoids with soluble epoxide hydrolase inhibitory activity from Callistemon citrinus.
Fitoterapia 109 , 39-44, (2016) Phytochemical analysis of the leaves and stems of Callistemon citrinus (Curtis) Skeels led to the isolation of two new alkylphloroglucinols, gallomyrtucommulone E and F (1 and 2), along with four othe... |
|
|
Therapeutic effects of the soluble epoxide hydrolase (sEH) inhibitor AUDA on atherosclerotic diseases.
Pharmazie 70(1) , 24-8, (2015) In this study, we aimed to detect the effects of the soluble epoxide hydrolase (sEH) inhibitor 12-(3-adamantan-1-yl-ureido)-dodecanoic acid (AUDA) on atherosclerotic diseases and to explore its mechan... |
| 12-[[(tricyclo[3.3.1.13,7]dec-1-ylamino)carbonyl]amino]-dodecanoic acid |
| HMS2204E15 |
| 12-[(Adamantan-1-ylcarbamoyl)amino]dodecanoic acid |
| 12-{[(3s,5s,7s)-Adamantan-1-ylcarbamoyl]amino}dodecanoic acid |
| Dodecanoic acid, 12-[[(tricyclo[3.3.1.1]dec-1-ylamino)carbonyl]amino]- |
| 12-(3-adamantan-1-ylureido)dodecanoic acid |
| Urea-based compound,18 |
| 12-[(tricyclo[3.3.1.1]dec-1-ylcarbamoyl)amino]dodecanoic acid |
| AUDA |