CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
KG8000000
-
CHEMICAL NAME :
-
Estr-4-en-3-one, 17-beta-hydroxy-17-alpha-methyl-
-
CAS REGISTRY NUMBER :
-
514-61-4
-
BEILSTEIN REFERENCE NO. :
-
2622263
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
21
-
MOLECULAR FORMULA :
-
C19-H28-O2
-
MOLECULAR WEIGHT :
-
288.47
-
WISWESSER LINE NOTATION :
-
L E5 B666 OV MUTJ E1 FQ F1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
45 gm/kg/30D-C
-
TOXIC EFFECTS :
-
Endocrine - adrenal cortex hypoplasia Endocrine - other changes Blood - other changes
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 11,571,1977
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
9360 mg/kg/26W-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in bladder weight Endocrine - other changes Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
REFERENCE :
-
KSRNAM Kiso to Rinsho. Clinical Report. (Yubunsha Co., Ltd., 1-5, Kanda Suda-Cho, Chiyoda-ku, KS Bldg., Tokyo 101, Japan) V.1- 1960- Volume(issue)/page/year: 11,588,1977 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
18 mg/kg
-
SEX/DURATION :
-
female 12-24 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord) Reproductive - Specific Developmental Abnormalities - urogenital system
-
REFERENCE :
-
BRGOAY Bulletin de la Societe Royale Belge de Gynecologie et d'Obstetrique. (Brussels, Belgium) V.1-39, 1925-69. Discontinued. Volume(issue)/page/year: 28,137,1958
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
20 mg/kg
-
SEX/DURATION :
-
female 6-9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 15,955,1978
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
200 mg/kg
-
SEX/DURATION :
-
female 6-9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 15,955,1978
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
14 mg/kg
-
SEX/DURATION :
-
male 7 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - prostate, seminal vesicle, Cowper's gland, accessory glands Reproductive - Paternal Effects - other effects on male
-
REFERENCE :
-
ACENA7 Acta Endocrinologica (Copenhagen). (Periodica, Skolegade 12 E, DK-2500 Valby, Denmark) V.1- 1948- Volume(issue)/page/year: 22,318,1956
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
112 mg/kg
-
SEX/DURATION :
-
male 14 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct
-
REFERENCE :
-
ANYAA9 Annals of the New York Academy of Sciences. (New York Academy of Sciences, 2 E. 63rd St., New York, NY 10021) V.1- 1877- Volume(issue)/page/year: 71,500,1958
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 12 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - menstrual cycle changes or disorders
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 29,95,1979
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
17500 ug/kg
-
SEX/DURATION :
-
female 15-21 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - urogenital system
-
REFERENCE :
-
ACENA7 Acta Endocrinologica (Copenhagen). (Periodica, Skolegade 12 E, DK-2500 Valby, Denmark) V.1- 1948- Volume(issue)/page/year: 38,128,1961
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
2 mg/kg
-
SEX/DURATION :
-
female 6-9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 15,955,1978
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
20 mg/kg
-
SEX/DURATION :
-
female 6-9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 15,955,1978
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
150 mg/kg
-
SEX/DURATION :
-
female 30 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
REFERENCE :
-
RPHRA6 Recent Progress In Hormone Research. Proceedings of the Laurentian Hormone Conference. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1947- Volume(issue)/page/year: 20,395,1964
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
20 mg/kg
-
SEX/DURATION :
-
female 6-7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 15,955,1978
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
200 mg/kg
-
SEX/DURATION :
-
female 6-7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 15,955,1978
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1600 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
REFERENCE :
-
85GRAA "The Control of Fertility," Pincus, G., New York, Academic Press, 1965 Volume(issue)/page/year: -,150,1965
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
2 mg/kg
-
SEX/DURATION :
-
female 6-7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 15,955,1978
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
20 mg/kg
-
SEX/DURATION :
-
female 6-7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
OYYAA2 Oyo Yakuri. Pharmacometrics. (Oyo Yakuri Kenkyukai, CPO Box 180, Sendai 980-91, Japan) V.1- 1967- Volume(issue)/page/year: 15,955,1978
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
1280 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
REFERENCE :
-
85GRAA "The Control of Fertility," Pincus, G., New York, Academic Press, 1965 Volume(issue)/page/year: -,150,1965
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
200 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
REFERENCE :
-
85GRAA "The Control of Fertility," Pincus, G., New York, Academic Press, 1965 Volume(issue)/page/year: -,57,1965
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 5 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
REFERENCE :
-
ANREAK Anatomical Record. (Alan R. Liss, Inc., 41 E. 11th St., New York, NY 10003) V.1- 1906/08- Volume(issue)/page/year: 142,469,1962
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
50 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
REFERENCE :
-
ENDOAO Endocrinology (Baltimore). (Williams & Wilkins Co., 428 E. Preston St., Baltimore, MD 21203) V.1- 1917- Volume(issue)/page/year: 59,695,1956
|
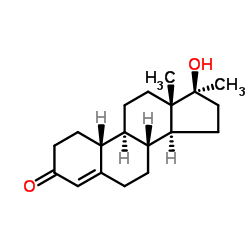





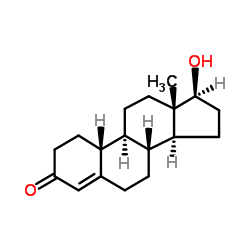
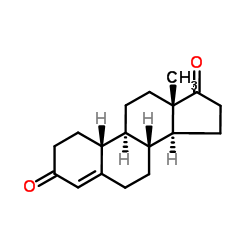
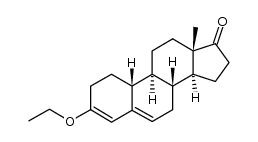

 CAS#:302-76-1
CAS#:302-76-1 CAS#:7100-20-1
CAS#:7100-20-1 CAS#:153-23-1
CAS#:153-23-1