L-(+)-ERYTHROSE

L-(+)-ERYTHROSE structure
|
Common Name | L-(+)-ERYTHROSE | ||
|---|---|---|---|---|
| CAS Number | 533-49-3 | Molecular Weight | 120.10400 | |
| Density | 1.41 g/cm3 | Boiling Point | 311.1ºC at 760 mmHg | |
| Molecular Formula | C4H8O4 | Melting Point | 164ºC | |
| MSDS | USA | Flash Point | 156.2ºC | |
Use of L-(+)-ERYTHROSEL-Erythrose is an aldose carbohydrate that has been used in glycation studies and to characterize erythrose reductase activity. |
| Name | l-(+)-erythrose |
|---|---|
| Synonym | More Synonyms |
| Description | L-Erythrose is an aldose carbohydrate that has been used in glycation studies and to characterize erythrose reductase activity. |
|---|---|
| Related Catalog |
| Density | 1.41 g/cm3 |
|---|---|
| Boiling Point | 311.1ºC at 760 mmHg |
| Melting Point | 164ºC |
| Molecular Formula | C4H8O4 |
| Molecular Weight | 120.10400 |
| Flash Point | 156.2ºC |
| Exact Mass | 120.04200 |
| PSA | 77.76000 |
| Index of Refraction | 1.505 |
| Storage condition | 2~8°C |
Synonym: Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
Risk Phrases: None Listed. Section 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW
Not available. Potential Health Effects Eye: May cause eye irritation. Skin: May cause skin irritation. May be harmful if absorbed through the skin. Ingestion: May cause irritation of the digestive tract. May be harmful if swallowed. Inhalation: May cause respiratory tract irritation. May be harmful if inhaled. Chronic: Not available. Section 4 - FIRST AID MEASURES Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Skin: Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Ingestion: Get medical aid. Wash mouth out with water. Inhalation: Remove from exposure and move to fresh air immediately. Notes to Physician: Treat symptomatically and supportively. Section 5 - FIRE FIGHTING MEASURES General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Extinguishing Media: Use water spray, dry chemical, carbon dioxide, or chemical foam. Section 6 - ACCIDENTAL RELEASE MEASURES General Information: Use proper personal protective equipment as indicated in Section 8. Spills/Leaks: Vacuum or sweep up material and place into a suitable disposal container. Section 7 - HANDLING and STORAGE Handling: Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes. Storage: Store in a cool, dry place. Store in a tightly closed container. Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION Engineering Controls: Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 533-49-3: Personal Protective Equipment Eyes: Not available. Skin: Wear appropriate protective gloves to prevent skin exposure. Clothing: Wear appropriate protective clothing to prevent skin exposure. Respirators: Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced. Section 9 - PHYSICAL AND CHEMICAL PROPERTIES Physical State: Clear liquid Color: light yellow Odor: Not available. pH: Not available. Vapor Pressure: Not available. Viscosity: Not available. Boiling Point: Not available. Freezing/Melting Point: 164 deg C Autoignition Temperature: Not available. Flash Point: > 230 deg C (> 446.00 deg F) Explosion Limits, lower: Not available. Explosion Limits, upper: Not available. Decomposition Temperature: Solubility in water: Soluble. Specific Gravity/Density: Molecular Formula: C4H8O4 Molecular Weight: 120.11 Section 10 - STABILITY AND REACTIVITY Chemical Stability: Stable under normal temperatures and pressures. Conditions to Avoid: Incompatible materials, excess heat. Incompatibilities with Other Materials: Strong oxidizing agents. Hazardous Decomposition Products: Carbon monoxide, carbon dioxide. Hazardous Polymerization: Has not been reported Section 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 533-49-3 unlisted. LD50/LC50: Not available. Carcinogenicity: L(+)-Erythrose - Not listed by ACGIH, IARC, or NTP. Section 12 - ECOLOGICAL INFORMATION Section 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations. Section 14 - TRANSPORT INFORMATION IATA Shipping Name: Not regulated. Hazard Class: UN Number: Packing Group: IMO Shipping Name: Not regulated. Hazard Class: UN Number: Packing Group: RID/ADR Not regulated as a hazardous material. Section 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives Hazard Symbols: Not available. Risk Phrases: Safety Phrases: S 24/25 Avoid contact with skin and eyes. WGK (Water Danger/Protection) CAS# 533-49-3: No information available. Canada None of the chemicals in this product are listed on the DSL/NDSL list. CAS# 533-49-3 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 533-49-3 is not listed on the TSCA inventory. It is for research and development use only. SECTION 16 - ADDITIONAL INFORMATION N/A |
| Precursor 9 | |
|---|---|
| DownStream 3 | |
| HS Code | 2912491000 |
|---|---|
| Summary | 2912491000. other aldehyde-alcohols. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
Theoretical study of the mutarotation of erythrose and threose: acid catalysis.
Carbohydr. Res. 372 , 1-8, (2013) The acid catalysis of the mutarotation mechanism in the two aldotetroses, d-erythrose and d-threose, has been studied at B3LYP/6-311++G(d,p) computational level in gas phase and in solution employing ... |
|
|
Mineral supplementation increases erythrose reductase activity in erythritol biosynthesis from glycerol by Yarrowia lipolytica.
Appl. Biochem. Biotechnol. 172(6) , 3069-78, (2014) The aim of this study was to examine the impact of divalent copper, iron, manganese, and zinc ions on the production of erythritol from glycerol by Yarrowia lipolytica and their effect on the activity... |
|
|
Production of L-erythrose via L-erythrulose from erythritol using microbial and enzymatic reactions.
J. Biosci. Bioeng. 92 , 237-241, (2001) A rare aldotetrose, L-erythrose, was produced from erythritol via a two-step reaction. In the first step, complete oxidation of erythritol to L-erythrulose was achieved by using Gluconobacter frateuri... |
| EINECS 208-567-8 |
| MFCD00064377 |
| D-erythrose |
| L-erythro-2,3,4-Trihydroxy-butyraldehyd |
| (2S,3S)-2,3,4-Trihydroxybutanal |
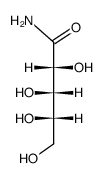 CAS#:74421-66-2
CAS#:74421-66-2 CAS#:35831-80-2
CAS#:35831-80-2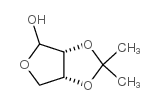 CAS#:23262-84-2
CAS#:23262-84-2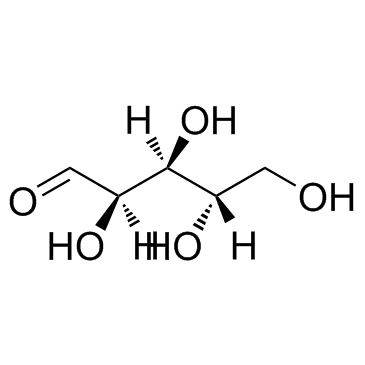 CAS#:5328-37-0
CAS#:5328-37-0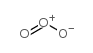 CAS#:10028-15-6
CAS#:10028-15-6 CAS#:152202-52-3
CAS#:152202-52-3 CAS#:25546-40-1
CAS#:25546-40-1 CAS#:64283-40-5
CAS#:64283-40-5 CAS#:3555-01-9
CAS#:3555-01-9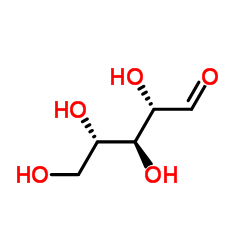 CAS#:24259-59-4
CAS#:24259-59-4![[[(1E)-1-(carbamothioylhydrazinylidene)-3,4-dihydroxy-butan-2-ylidene]amino]thiourea structure](https://image.chemsrc.com/caspic/375/54097-86-8.png) CAS#:54097-86-8
CAS#:54097-86-8
