Boc-Nva-OH
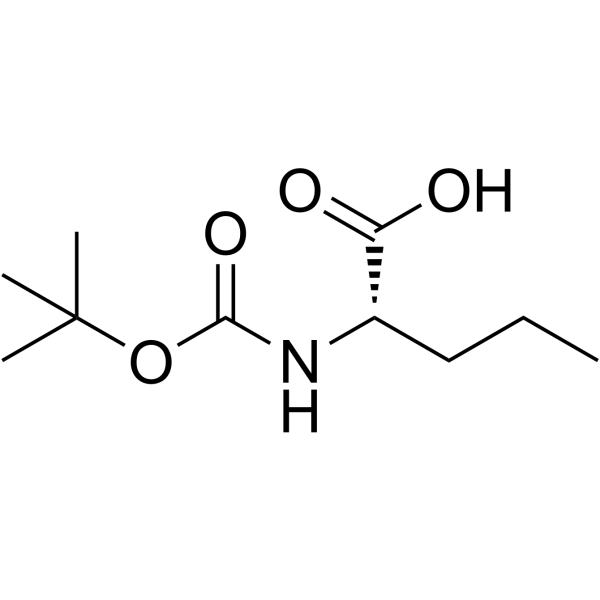
Boc-Nva-OH structure
|
Common Name | Boc-Nva-OH | ||
|---|---|---|---|---|
| CAS Number | 53308-95-5 | Molecular Weight | 217.262 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 348.3±25.0 °C at 760 mmHg | |
| Molecular Formula | C10H19NO4 | Melting Point | 43-47ºC | |
| MSDS | Chinese USA | Flash Point | 164.4±23.2 °C | |
Use of Boc-Nva-OHBoc-Nva-OH is a valine derivative[1]. |
| Name | (2S)-2-[(2-methylpropan-2-yl)oxycarbonylamino]pentanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Boc-Nva-OH is a valine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 348.3±25.0 °C at 760 mmHg |
| Melting Point | 43-47ºC |
| Molecular Formula | C10H19NO4 |
| Molecular Weight | 217.262 |
| Flash Point | 164.4±23.2 °C |
| Exact Mass | 217.131409 |
| PSA | 75.63000 |
| LogP | 2.16 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.462 |
| Storage condition | 0-6°C |
|
~67% 
Boc-Nva-OH CAS#:53308-95-5 |
| Literature: Naskar, Jishu; Roy, Subhasish; Joardar, Anindita; Das, Sumantra; Banerjee, Arindam Organic and Biomolecular Chemistry, 2011 , vol. 9, # 19 p. 6610 - 6615 |
|
~98% 
Boc-Nva-OH CAS#:53308-95-5 |
| Literature: Tetrahedron Asymmetry, , vol. 17, # 13 p. 1995 - 1999 |
|
~% 
Boc-Nva-OH CAS#:53308-95-5 |
| Literature: Tetrahedron Letters, , vol. 28, # 48 p. 6069 - 6072 |
|
~% 
Boc-Nva-OH CAS#:53308-95-5 |
| Literature: Tetrahedron Letters, , vol. 28, # 48 p. 6069 - 6072 |
|
~% 
Boc-Nva-OH CAS#:53308-95-5 |
| Literature: Tetrahedron Letters, , vol. 28, # 48 p. 6069 - 6072 |
|
~% 
Boc-Nva-OH CAS#:53308-95-5 |
| Literature: Tetrahedron Letters, , vol. 28, # 48 p. 6069 - 6072 |
|
~% 
Boc-Nva-OH CAS#:53308-95-5 |
| Literature: Tetrahedron Letters, , vol. 28, # 48 p. 6069 - 6072 |
|
~% 
Boc-Nva-OH CAS#:53308-95-5 |
| Literature: Tetrahedron Letters, , vol. 28, # 48 p. 6069 - 6072 |
|
~% 
Boc-Nva-OH CAS#:53308-95-5 |
| Literature: Tetrahedron, , vol. 47, # 29 p. 5453 - 5462 |
| HS Code | 2924199090 |
|---|---|
| Summary | 2924199090. other acyclic amides (including acyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Transport and signaling via the amino acid binding site of the yeast Gap1 amino acid transceptor.
Nat. Chem. Biol. 5 , 45-52, (2009) Transporter-related nutrient sensors, called transceptors, mediate nutrient activation of signaling pathways through the plasma membrane. The mechanism of action of transporting and nontransporting tr... |
| BOC-L-NVA-OH |
| (2S)-2-({[(2-Methyl-2-propanyl)oxy]carbonyl}amino)pentanoic acid |
| N-(tert-Butoxycarbonyl)-L-norvaline |
| MFCD00037268 |
| BOC-NVA-OH |
| L-Norvaline, N-[(1,1-dimethylethoxy)carbonyl]- |
| Boc-L-Norvaline |
| N-BOC-L-NORVALINE |
| RARECHEM EM WB 0170 |
| BOC-NORVALINE |
| Boc-L-norvaline-OH |
| N-{[(2-Methyl-2-propanyl)oxy]carbonyl}-L-norvaline |
| BOC-1-NORVALINE |
| N-[(1,1-Dimethylethoxy)carbonyl]-L-methionine |

![D-Norvaline, N-[(1,1-dimethylethoxy)carbonyl]-, Methyl ester structure](https://image.chemsrc.com/caspic/001/116611-58-6.png)
![N-[(1R)-1-(Hydroxymethyl)butyl]carbamic acid 1,1-dimethylethyl ester structure](https://image.chemsrc.com/caspic/039/116611-57-5.png)

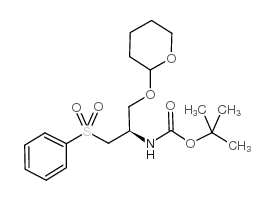

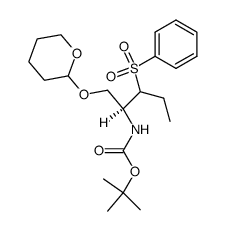
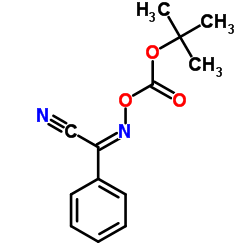
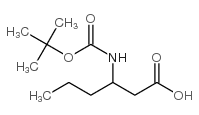 CAS#:209398-26-5
CAS#:209398-26-5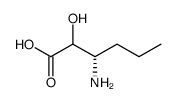 CAS#:402959-32-4
CAS#:402959-32-4
