Sucralfate
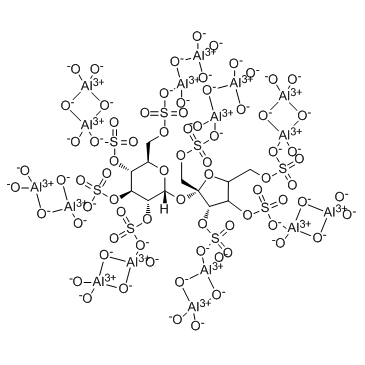
Sucralfate structure
|
Common Name | Sucralfate | ||
|---|---|---|---|---|
| CAS Number | 54182-58-0 | Molecular Weight | 2046.42 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C12H14Al16O75S8 | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
Use of SucralfateSucralfate is a cytoprotective agent which has been employed for prevention and treatment of several gastrointestinal diseases. |
| Name | Sucralfate |
|---|---|
| Synonym | More Synonyms |
| Description | Sucralfate is a cytoprotective agent which has been employed for prevention and treatment of several gastrointestinal diseases. |
|---|---|
| Related Catalog | |
| In Vivo | Sucralfate is a cytoprotective agent which has been employed for prevention and treatment of several gastrointestinal diseases. Enemas containing Sucralfate improves the inflammation and increases the tissue contents of neutral and acid mucins. The content of neutral mucins does not change with the time or concentration of Sucralfate used, while acid mucins increases with concentration and time of intervention. A significant increase in tissue content of neutral mucins in animals subjected to irrigation with Sucralfate (SCF) is found compare to animals irrigated with S.F. 0.9%, regardless of the concentration and duration of intervention[1]. |
| Animal Admin | Thirty-six male Wistar rats (300 to 350 g) are used in this study. The animals are divided into two experimental groups with 18 animals in each group. Each experimental group is divided into six subgroups (n=6) according to the intervention solution employed and time of intervention. In the first and second subgroups, 12 animals receive daily rectal enemas containing 40 mL of 0.9% saline solution (control subgroup) at 37°C for two weeks (n=6) and four weeks (n=6). In the second subgroup, 12 animals receive daily rectal enemas containing 40 mL of Sucralfate (SCF) at a concentration of 1.0 g/kg for two weeks (n=6) and four weeks (n=6). Finally, 12 animals of the third subgroup receive daily enemas containing 40 mL of Sucralfate at a concentration of 2.0 g/kg for two weeks (n=6) and four weeks (n=6). In order to standardize the speed and time of application, the enemas are administered in all animals with an infusion pump whose speed is standardized at 2/mL/min[1]. |
| References |
| Molecular Formula | C12H14Al16O75S8 |
|---|---|
| Molecular Weight | 2046.42 |
| PSA | 1435.37000 |
| Storage condition | 2-8°C |
| Water Solubility | Practically insoluble in water, in ethanol (96 per cent) and in methylene chloride. It dissolves in dilute solutions of mineral acids and alkali hydroxides. |
|
Section 1. Chemical Product and Company Identification Sucralfate Common Name/ Trade Name Sucralfate Section 4. First Aid Measures Eye ContactCheck for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water for at
least 15 minutes. Get medical attention if irritation occurs. Skin ContactWash with soap and water. Cover the irritated skin with an emollient. Get medical attention if irritation develops. Serious Skin ContactNot available. InhalationIf inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical attention. Serious InhalationNot available. IngestionDo NOT induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Loosen tight clothing such as a collar, tie, belt or waistband. Get medical attention if symptoms appear. Serious IngestionNot available. Section 5. Fire and Explosion Data Flammability of the Product May be combustible at high temperature. Auto-Ignition Temperature Not available. Flash PointsNot available. Flammable LimitsNot available. Products of CombustionThese products are carbon oxides (CO, CO2), sulfur oxides (SO2, SO3...). Some metallic oxides. Fire Hazards in Presence of Slightly flammable to flammable in presence of heat. Various Substances Explosion Hazards in Presence Risks of explosion of the product in presence of mechanical impact: Not available. Risks of explosion of the product in presence of static discharge: Not available. of Various Substances SMALL FIRE: Use DRY chemical powder. Fire Fighting Media and InstructionsLARGE FIRE: Use water spray, fog or foam. Do not use water jet. As with most organic solids, fire is possible at elevated temperatures Special Remarks on Fire Hazards Special Remarks on Explosion Fine dust dispersed in air in sufficient concentrations, and in the presence of an ignition source is a potential dust Hazardsexplosion hazard. Section 6. Accidental Release Measures Small SpillUse appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and dispose of according to local and regional authority requirements. Large SpillUse a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water on the contaminated surface and allow to evacuate through the sanitary system. Sucralfate Section 7. Handling and Storage PrecautionsKeep away from heat. Keep away from sources of ignition. Ground all equipment containing material. Do not ingest. Do not breathe dust. If ingested, seek medical advice immediately and show the container or the label. Keep away from incompatibles such as oxidizing agents. StorageKeep container tightly closed. Keep container in a cool, well-ventilated area. Section 8. Exposure Controls/Personal Protection Engineering ControlsUse process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to airborne contaminants below the exposure limit. Personal ProtectionSafety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves. Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used a Large Spillto avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist BEFORE handling this product. Exposure LimitsNot available. Section 9. Physical and Chemical Properties Physical state and appearance Solid. (Amorphous solid powder or lumps.)OdorOdorless. TasteNot available. Molecular Weight2086.7 g/mole ColorWhite. pH (1% soln/water)Not applicable. Not available. Boiling Point Melting PointNot available. Not available. Critical Temperature Specific GravityNot available. Vapor PressureNot applicable. Vapor DensityNot available. VolatilityNot available. Odor ThresholdNot available. Water/Oil Dist. Coeff.Not available. Ionicity (in Water)Not available. Dispersion PropertiesNot available. SolubilityInsoluble in cold water. Practically insoluble in ethanol, chloroform. Soluble in dilute hydrochloric acid and sodium hydroxide solution. Section 10. Stability and Reactivity Data StabilityThe product is stable. Instability TemperatureNot available. Conditions of InstabilityExcess heat, incompatible materials Incompatibility with various Reactive with oxidizing agents. substances Sucralfate Non-corrosive in presence of glass. Corrosivity Special Remarks onNot available. Reactivity Special Remarks onNot available. Corrosivity Will not occur. Polymerization Section 11. Toxicological Information Routes of EntryInhalation. Ingestion. Toxicity to AnimalsAcute oral toxicity (LD50): >8000 mg/kg [Mouse]. Chronic Effects on Humans Not available. Other Toxic Effects onSlightly hazardous in case of skin contact (irritant), of ingestion, of inhalation. Humans Special Remarks onNot available. Toxicity to Animals Special Remarks onNot available. Chronic Effects on Humans Special Remarks on otherAcute Potential Health Effects: Toxic Effects on HumansSkin: May cause skin irritation. Eyes: May cause eye irritation. Inhalation: May cause respiratory tract irritation. Ingestion: Low Hazard. It is not readily absorbed into the body. May cause abdominal pain/stomach cramps, indigestion, gas (flatulence), constipation. Other less common or rare symptoms may include diarrhea, headache, drowsiness, vertigo, dizziness or lightheadness, dry mouth, nausea, skin rash, hives or itiching, back pain, Medical Conditions Aggravated by Exposure: Gastrointestinal tract obstruction disease. Sucralfate may bind with other foods and drugs and cause obstruction of the gastrointestinal tract; Kidney failure or decreased kidney function. Use may leaed to a toxic increase of aluminum blood levels; Use of the following drugs: Ciprofloxacin, Digoxin, Norfloxacin, Ofloxacin, Phenytoin, Theophyplline Ketoconazole, Thyroxine, Warfarin, Lansoprazole, Amitriptyline. Sucralfate may prevent these medicines from working properly. Section 12. Ecological Information EcotoxicityNot available. BOD5 and CODNot available. Products of BiodegradationPossibly hazardous short term degradation products are not likely. However, long term degradation products may arise. The product itself and its products of degradation are not toxic. Toxicity of the Products of Biodegradation Special Remarks on theNot available. Products of Biodegradation Section 13. Disposal Considerations Waste DisposalWaste must be disposed of in accordance with federal, state and local environmental control regulations. Sucralfate Section 14. Transport Information DOT ClassificationNot a DOT controlled material (United States). IdentificationNot applicable. Not applicable. Special Provisions for Transport DOT (Pictograms) Section 15. Other Regulatory Information and Pictograms No products were found. Federal and State Regulations CaliforniaCalifornia prop. 65: This product contains the following ingredients for which the State of California has found to cause cancer which would require a warning under the statute: No products were found. Proposition 65 Warnings California prop. 65: This product contains the following ingredients for which the State of California has found to cause birth defects which would require a warning under the statute: No products were found. Other RegulationsEINECS: This product is on the European Inventory of Existing Commercial Chemical Substances. Other ClassificationsWHMIS (Canada) Not controlled under WHMIS (Canada). DSCL (EEC)This product is not classified according Not applicable. to the EU regulations. Health Hazard HMIS (U.S.A.)1 National Fire Protection 1 Flammability 1 Association (U.S.A.) Fire Hazard 1 0 Reactivity Health Reactivity 0 Specific hazard Personal Protection E WHMIS (Canada) (Pictograms) DSCL (Europe) (Pictograms) TDG (Canada) (Pictograms) ADR (Europe) (Pictograms) Sucralfate Protective Equipment Gloves. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | BD0900000 |
| HS Code | 29400090 |
|
Anti-secretory and cyto-protective effects of chebulinic acid isolated from the fruits of Terminalia chebula on gastric ulcers.
Phytomedicine 20(6) , 506-11, (2013) In continuation of our drug discovery program on Indian medicinal plants, the gastro protective mechanism of chebulinic acid isolated from Terminalia chebula fruit was investigated. Chebulinic acid wa... |
|
|
Protective effect of Calamintha officinalis Moench leaves against alcohol-induced gastric mucosa injury in rats. Macroscopic, histologic and phytochemical analysis.
Phytother Res. 26(6) , 839-44, (2012) Calamintha officinalis Moench (Lamiaceae) is an aromatic plant used since ancient times for its preservative and medicinal properties. The plant, known as 'Mentuccia' in Central Italy, is used in cook... |
|
|
Systematic review: Antacids, H2-receptor antagonists, prokinetics, bismuth and sucralfate therapy for non-ulcer dyspepsia.
Aliment. Pharmacol. Ther. 17(10) , 1215-27, (2003) Evidence for the effectiveness of antacids, histamine-2 receptor antagonists, bismuth salts, sucralfate and prokinetic therapy in non-ulcer dyspepsia is conflicting.To conduct a systematic review eval... |
| Venter |
| Urbal |
| Sucrate |
| Succosa |
| Keal |
| Citogel |
| ulcerban |
| carafate |
| Ulcar |
| Sugast |