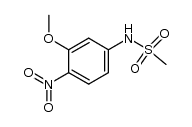
acridinyl anisidide

acridinyl anisidide structure
|
Common Name | acridinyl anisidide | ||
|---|---|---|---|---|
| CAS Number | 54301-15-4 | Molecular Weight | 429.92000 | |
| Density | N/A | Boiling Point | 563ºC at 760 mmHg | |
| Molecular Formula | C21H20ClN3O3S | Melting Point | 197-199ºC | |
| MSDS | Chinese USA | Flash Point | 294.3ºC | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of acridinyl anisidideAmsacrine is an inhibitor of topoisomerase II, and acts as an antineoplastic agent which can intercalates into the DNA of tumor cells. |
| Name | Amsacrine hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Amsacrine is an inhibitor of topoisomerase II, and acts as an antineoplastic agent which can intercalates into the DNA of tumor cells. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase II |
| In Vitro | Amsacrine blocks HERG currents in HEK 293 cells and Xenopus oocytes in a concentration-dependent manner, with IC50 values of 209.4 nM and 2.0 μM, respectively. Amsacrine causes a negative shift in the voltage dependence of both activation (−7.6 mV) and inactivation (−7.6 mV). HERG current block by amsacrine is not frequency dependent[1]. In vitro studies of normal human lymphocytes with various concentrations of m-AMSA, show both increased levels of chromosomal aberrations, ranging from 8% to 100%, and increase SCEs, ranging from 1.5 times the normal at the lowest concentration studied (0.005 μg/mL) to 12 times the normal (0.25 μg/mL)[3]. Amsacrine-induced apoptosis of U937 cells is characterized by caspase-9 and caspase-3 activation, increased intracellular Ca2+ concentration, mitochondrial depolarization, and MCL1 down-regulation. Amsacrine induces MCL1 down-regulation by decreasing its stability. Further, amsacrine-treated U937 cells show AKT degradation and Ca2+-mediated ERK inactivation[4]. |
| In Vivo | In animals treated with different doses of amsacrine (0.5-12 mg/kg), the frequencies of micronucleated polychromatic erythrocytes increase significantly after treatment with 9 and 12 mg/kg. Furthermore, the present study demonstrates for the first time that amsacrine has high incidences of clastogenicity and low incidences of aneugenicity whereas nocodazole has high incidences of aneugenicity and low incidences of clastogenicity during mitotic phases in vivo[2]. |
| Animal Admin | Mice[2] Amsacrine is investigated in three separated experiments. In the first experiment, animals are treated by intraperitoneal injection with 0.5, 1.5 and 4.5 mg/kg of Amsacrine and bone marrow is sampled 24 h after treatment. Preliminary negative MN results at this sampling time lead to the use of 30 h sampling time for Amsacrine. Thus, in the second experiment, mice are treated with 0.5, 1.5 and 4.5 mg/kg of Amsacrine and bone marrow is sampled 30 h after treatment. The doses and sampling times for Amsacrine are chosen by reference to earlier studies and the selected doses are within the dose range used for human chemotherapy. The results again show that the micronuclei frequency in the bone marrow of mice is not affected by treatment with any of the selected doses of the test agent, at 30 h sampling time, thus, in the third experiment, mice are treated with 6, 9 and 12 mg/kg of Amsacrine and bone marrow is sampled 24 and 30 h after treatment. |
| References |
| Boiling Point | 563ºC at 760 mmHg |
|---|---|
| Melting Point | 197-199ºC |
| Molecular Formula | C21H20ClN3O3S |
| Molecular Weight | 429.92000 |
| Flash Point | 294.3ºC |
| Exact Mass | 429.09100 |
| PSA | 88.70000 |
| LogP | 6.54050 |
| InChIKey | WDISRLXRMMTXEV-UHFFFAOYSA-N |
| SMILES | COc1cc(NS(C)(=O)=O)ccc1Nc1c2ccccc2nc2ccccc12.Cl |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H315-H319-H335 |
| Precautionary Statements | P261-P301 + P310-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic; |
| Risk Phrases | R25;R36/37/38 |
| Safety Phrases | S26-S45 |
| RIDADR | UN 2811 |
| WGK Germany | 3.0 |
| RTECS | PB1081000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2935009090 |
|
~% 
acridinyl anisidide CAS#:54301-15-4 |
| Literature: Journal of Medicinal Chemistry, , vol. 30, # 4 p. 652 - 658 |
|
~% 
acridinyl anisidide CAS#:54301-15-4 |
| Literature: Journal of Medicinal Chemistry, , vol. 30, # 4 p. 652 - 658 |
|
~% 
acridinyl anisidide CAS#:54301-15-4 |
| Literature: Journal of Medicinal Chemistry, , vol. 30, # 4 p. 652 - 658 |
|
~% 
acridinyl anisidide CAS#:54301-15-4 |
| Literature: Journal of Medicinal Chemistry, , vol. 30, # 4 p. 652 - 658 |
| HS Code | 2935009090 |
|---|---|
| Summary | 2935009090 other sulphonamides VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:35.0% |
|
Aneuploidy induces profound changes in gene expression, proliferation and tumorigenicity of human pluripotent stem cells.
Nat. Commun. 5 , 4825, (2014) Human pluripotent stem cells (hPSCs) tend to acquire genomic aberrations in culture, the most common of which is trisomy of chromosome 12. Here we dissect the cellular and molecular implications of th... |
|
|
Induction of chromosomal aberrations (unstable and stable) by inhibitors of topoisomerase II, m-AMSA and VP16, using conventional Giemsa staining and chromosome painting techniques.
Mutagenesis 13 , 39-43, (1998) Frequencies of symmetrical and asymmetrical exchange aberrations induced by two inhibitors of topoisomerase II, namely, 4'-(9-acridinylamino) methanesulfon-m-anisidide (m-AMSA) and etoposide (VP16), w... |
|
|
Mechanism of antitumor drug action: poisoning of mammalian DNA topoisomerase II on DNA by 4'-(9-acridinylamino)-methanesulfon-m-anisidide.
Proc. Natl. Acad. Sci. U. S. A. 81 , 1361-1365, (1984) The intercalative acridine derivative 4'-(9-acridinylamino)methanesulfon-m-anisidide (m-AMSA), but not its isomer o-AMSA, is a potent antitumor drug that in mammalian cells stimulates the formation of... |
| N-[4-(acridin-9-ylamino)-3-methoxyphenyl]methanesulfonamide,hydrochloride |
| Amsacrine (hydrochloride) |