Quinoline-2-carboxaldehyde
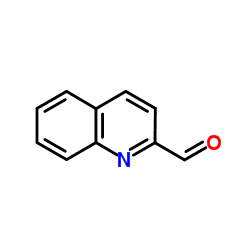
Quinoline-2-carboxaldehyde structure
|
Common Name | Quinoline-2-carboxaldehyde | ||
|---|---|---|---|---|
| CAS Number | 5470-96-2 | Molecular Weight | 157.17 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 314.3±15.0 °C at 760 mmHg | |
| Molecular Formula | C10H7NO | Melting Point | 66-71 ºC | |
| MSDS | Chinese USA | Flash Point | 151.9±27.8 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Quinoline-2-carboxaldehydeQuinoline-2-carboxaldehyde is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 2-Quinolinecarboxaldehyde |
|---|---|
| Synonym | More Synonyms |
| Description | Quinoline-2-carboxaldehyde is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 314.3±15.0 °C at 760 mmHg |
| Melting Point | 66-71 ºC |
| Molecular Formula | C10H7NO |
| Molecular Weight | 157.17 |
| Flash Point | 151.9±27.8 °C |
| Exact Mass | 157.052765 |
| PSA | 29.96000 |
| LogP | 2.14 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.687 |
| InChIKey | WPYJKGWLDJECQD-UHFFFAOYSA-N |
| SMILES | O=Cc1ccc2ccccc2n1 |
| Storage condition | Refrigerator (+4°C) |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S37/39-S26 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933499090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933499090 |
|---|---|
| Summary | 2933499090. other compounds containing in the structure a quinoline or isoquinoline ring-system (whether or not hydrogenated), not further fused. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Time-of-flight accurate mass spectrometry identification of quinoline alkaloids in honey.
Anal. Bioanal. Chem 407 , 6159-70, (2015) Time-of-flight accurate mass spectrometry (TOF-MS), following a previous chromatographic (gas or liquid chromatography) separation step, is applied to the identification and structural elucidation of ... |
|
|
Mixed ligand copper(II) complexes of 1,10-phenanthroline with tridentate phenolate/pyridyl/(benz)imidazolyl Schiff base ligands: covalent vs non-covalent DNA binding, DNA cleavage and cytotoxicity.
J. Inorg. Biochem. 140 , 255-68, (2014) A series of copper(II) complexes of the types [Cu(L)(phen)](ClO4) 1-2, where HL is a tridentate ligand with two nitrogen and one oxygen donor atoms (2NO) such as 2-(2-(1H-benzimidazol-2-yl)ethyliminom... |
|
|
A sugar-quinoline fluorescent chemosensor for selective detection of Hg2+ ion in natural water.
Chem. Commun. (Camb.) (42) , 4392-4, (2006) A selective and sensitive fluorescent sensor for detection of Hg2+ in natural water was achieved by incorporating the well-known fluorophore quinoline group and a water-soluble D-glucosamine group wit... |
| EINECS 226-804-3 |
| MFCD00075032 |
| Chinolin-2-carbaldehyd |
| quinoline-2-carbaldehyde |
| 2-Quinolinecarboxaldehyde |
| 2-Quinolinecarbaldehyde |
| Quinaldaldehyde |
| quinoline-2-carboxaldehyde |
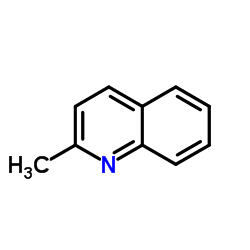 CAS#:91-63-4
CAS#:91-63-4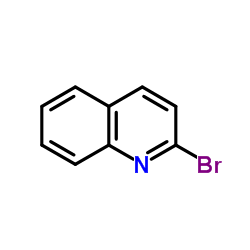 CAS#:2005-43-8
CAS#:2005-43-8 CAS#:201230-82-2
CAS#:201230-82-2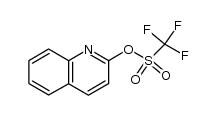 CAS#:109586-44-9
CAS#:109586-44-9 CAS#:5330-88-1
CAS#:5330-88-1 CAS#:67-56-1
CAS#:67-56-1 CAS#:1335204-96-0
CAS#:1335204-96-0 CAS#:53867-81-5
CAS#:53867-81-5 CAS#:4377-41-7
CAS#:4377-41-7 CAS#:79-46-9
CAS#:79-46-9 CAS#:5632-15-5
CAS#:5632-15-5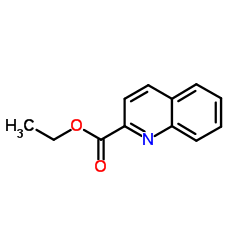 CAS#:4491-33-2
CAS#:4491-33-2![[1,2,3]TRIAZOLO[1,5-A]QUINOLINE structure](https://image.chemsrc.com/caspic/337/235-21-2.png) CAS#:235-21-2
CAS#:235-21-2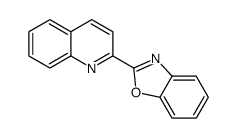 CAS#:24613-96-5
CAS#:24613-96-5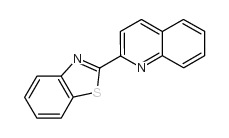 CAS#:24613-99-8
CAS#:24613-99-8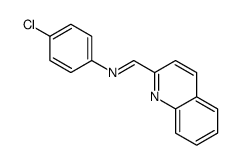 CAS#:24640-96-8
CAS#:24640-96-8 CAS#:76532-33-7
CAS#:76532-33-7 CAS#:772-03-2
CAS#:772-03-2 CAS#:14044-48-5
CAS#:14044-48-5 CAS#:93-10-7
CAS#:93-10-7
