AP-18
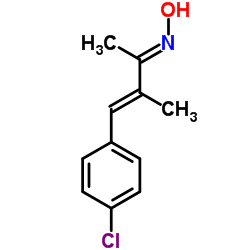
AP-18 structure
|
Common Name | AP-18 | ||
|---|---|---|---|---|
| CAS Number | 55224-94-7 | Molecular Weight | 209.672 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 348.4±34.0 °C at 760 mmHg | |
| Molecular Formula | C11H12ClNO | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 164.5±25.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of AP-18AP-18, a potent and selective TRPA1 inhibitor, blocks activation of TRPA1 by 50 μM Cinnamaldehyde with an IC50 of 3.1 μM and 4.5 μM for human and mouse TRPA1, respectively. AP-18 reverses complete Freund's adjuvant (CFA)-induced mechanical hyperalgesia in mice. AP-18 attenuated 30 μM AITC-induced Yo-Pro uptake in a concentration-dependent manner, with an IC50 of 10.3 μM[1][2][3]. |
| Name | 4-(4-Chlorophenyl)-3-methyl-3-buten-2-oneoxime |
|---|---|
| Synonym | More Synonyms |
| Description | AP-18, a potent and selective TRPA1 inhibitor, blocks activation of TRPA1 by 50 μM Cinnamaldehyde with an IC50 of 3.1 μM and 4.5 μM for human and mouse TRPA1, respectively. AP-18 reverses complete Freund's adjuvant (CFA)-induced mechanical hyperalgesia in mice. AP-18 attenuated 30 μM AITC-induced Yo-Pro uptake in a concentration-dependent manner, with an IC50 of 10.3 μM[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
TRPA1:~3 μM (IC50) |
| In Vitro | At concentrations up to 50 μM, AP-18 is unable to appreciably block activation of TRPV1, TRPV2, TRPV3, TRPV4, or TRPM8. AP-18 reversibly blocks mouse TRPA1 responses to iodoacetamide (an irreversible cysteine-alkylating agent) in CHO cells assayed by ratiometric Ca2+ imaging. AP-18 also blocks cold- and mustard-oil-induced activation of mouse TRPA1. AP-18 blocks cinnamaldehyde-induced TRPA1 currents in excised patches from Xenopus oocytes[1]. |
| In Vivo | AP18 (1 mM; injected in hindpaw of mice) significantly blocks cinnamaldehdye-induced but not capsaicin-induced nociceptive events, demonstrating efficacy and specificity[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 348.4±34.0 °C at 760 mmHg |
| Molecular Formula | C11H12ClNO |
| Molecular Weight | 209.672 |
| Flash Point | 164.5±25.7 °C |
| Exact Mass | 209.060745 |
| PSA | 32.59000 |
| LogP | 3.72 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.529 |
|
TRPA1 has a key role in the somatic pro-nociceptive actions of hydrogen sulfide.
PLoS ONE 7(10) , e46917, (2012) Hydrogen sulfide (H(2)S), which is produced endogenously from L-cysteine, is an irritant with pro-nociceptive actions. We have used measurements of intracellular calcium concentration, electrophysiolo... |
|
|
TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice.
PLoS ONE 4 , e7596, (2009) Nitric oxide (NO) can induce acute pain in humans and plays an important role in pain sensitization caused by inflammation and injury in animal models. There is evidence that NO acts both in the centr... |
|
|
A distinct role for transient receptor potential ankyrin 1, in addition to transient receptor potential vanilloid 1, in tumor necrosis factor α-induced inflammatory hyperalgesia and Freund's complete adjuvant-induced monarthritis.
Arthritis Rheum. 63 , 819-29, (2011) To investigate the involvement of transient receptor potential ankyrin 1 (TRPA1) in inflammatory hyperalgesia mediated by tumor necrosis factor α(TNFα) and joint inflammation.Mechanical hyperalgesia w... |
| (2E,3E)-4-(4-Chlorophenyl)-N-hydroxy-3-methyl-3-buten-2-imine |
| (2E,3E)-4-(4-Chlorophenyl)-N-hydroxy-3-methylbut-3-en-2-imine |
| 3-Buten-2-one, 4-(4-chlorophenyl)-3-methyl-, oxime, (2E,3E)- |
| N-Desmethylclozapine |
| 4-(4-chlorophenyl)-3-methylbut-3-en-2-one oxime |
| AP-18 |