Anhydroophiobolin A
Modify Date: 2025-08-25 12:47:39
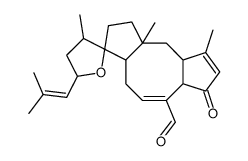
Anhydroophiobolin A structure
|
Common Name | Anhydroophiobolin A | ||
|---|---|---|---|---|
| CAS Number | 6026-65-9 | Molecular Weight | 382.53600 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C25H34O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Anhydroophiobolin AAnhydroophiobolin A is a potent inhibitor of photosynthesis with IC50s of 77 and 14 mM in the photosynthesis of chlorella and spinach, respectively. Anhydroophiobolin A is an analog of Ophiobolin A[1]. |
| Name | 3-Anhydrocochliobolin |
|---|---|
| Synonym | More Synonyms |
| Description | Anhydroophiobolin A is a potent inhibitor of photosynthesis with IC50s of 77 and 14 mM in the photosynthesis of chlorella and spinach, respectively. Anhydroophiobolin A is an analog of Ophiobolin A[1]. |
|---|---|
| Related Catalog | |
| References |
[1]. Jin-Myeon KIM, et al. Isolation of Ophiobolin A and Its Analogs as Inhibitors to Photosynthesis. |
| Molecular Formula | C25H34O3 |
|---|---|
| Molecular Weight | 382.53600 |
| Exact Mass | 382.25100 |
| PSA | 43.37000 |
| LogP | 5.21320 |
| 3-anhydroophiobolin a (14,17-epoxy-5-oxo-3,7,18-ophiobolatrien-21-al) |
| anhydroophiobolin a |