Gestodene
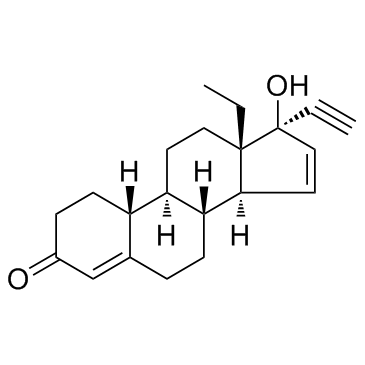
Gestodene structure
|
Common Name | Gestodene | ||
|---|---|---|---|---|
| CAS Number | 60282-87-3 | Molecular Weight | 310.430 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 462.7±45.0 °C at 760 mmHg | |
| Molecular Formula | C21H26O2 | Melting Point | 190-192°C | |
| MSDS | Chinese USA | Flash Point | 196.9±21.3 °C | |
| Symbol |

GHS08 |
Signal Word | Warning | |
Use of GestodeneGestodene(SHB 331;WL 70) is a progestogen hormonal contraceptive.Target: Estrogen Receptor/ERRGestodene is androgenically neutral, meaning that contraceptive pills containing gestodene do not exhibit the androgenic side effects (e.g. acne, hirsutism, weight gain) often associated with second-generation contraceptive pills, such as those containing levonorgestrel. When 40 micrograms of gestodene was taken, six out of seven women did not ovulate, and one out of seven had a cycle with luteal insufficiency. These data indicate that 40 micrograms of gestodene is the borderline dose for inhibition of ovulation. A combination of 75 micrograms gestodene with 30 micrograms ethinyl estradiol was found to inhibit ovulation in ten subjects, and no follicular maturation was noted [1]. gestodene bound with high affinity to the progesterone receptor, as did other synthetic and natural progestogens. However, gestodene did not bind to the estradiol receptor. The relative binding affinities of all tested synthetic and natural ligands showed no organ-specific differences and no differences between neoplastically transformed and normal tissues [2].Clinical indications: Female contraception |
| Name | Gestodene |
|---|---|
| Synonym | More Synonyms |
| Description | Gestodene(SHB 331;WL 70) is a progestogen hormonal contraceptive.Target: Estrogen Receptor/ERRGestodene is androgenically neutral, meaning that contraceptive pills containing gestodene do not exhibit the androgenic side effects (e.g. acne, hirsutism, weight gain) often associated with second-generation contraceptive pills, such as those containing levonorgestrel. When 40 micrograms of gestodene was taken, six out of seven women did not ovulate, and one out of seven had a cycle with luteal insufficiency. These data indicate that 40 micrograms of gestodene is the borderline dose for inhibition of ovulation. A combination of 75 micrograms gestodene with 30 micrograms ethinyl estradiol was found to inhibit ovulation in ten subjects, and no follicular maturation was noted [1]. gestodene bound with high affinity to the progesterone receptor, as did other synthetic and natural progestogens. However, gestodene did not bind to the estradiol receptor. The relative binding affinities of all tested synthetic and natural ligands showed no organ-specific differences and no differences between neoplastically transformed and normal tissues [2].Clinical indications: Female contraception |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 462.7±45.0 °C at 760 mmHg |
| Melting Point | 190-192°C |
| Molecular Formula | C21H26O2 |
| Molecular Weight | 310.430 |
| Flash Point | 196.9±21.3 °C |
| Exact Mass | 310.193268 |
| PSA | 37.30000 |
| LogP | 3.65 |
| Vapour Pressure | 0.0±2.6 mmHg at 25°C |
| Index of Refraction | 1.588 |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 10 | |
|---|---|
| DownStream 0 | |
|
Progestins in preventive hormone therapy. Including pharmacology of the new progestins, desogestrel, norgestimate, and gestodene: are there advantages?
Obstet. Gynecol. Clin. North Am. 21(2) , 299-319, (1994) Clofarabine was the latest new drug to be approved, in 2004, for relapsed or refractory acute lymphoblastic leukaemia (ALL). To investigate its value in the frontline treatment of ALL we applied clofa... |
|
|
Activation of NF-kappaB and COX-2 expression is associated with breakthrough bleeding in patients using oral contraceptives in extended regimens.
Gynecol. Endocrinol. 26(4) , 265-9, (2010) The objective of the present study was to determine whether there is an increase in endometrial inflammation associated with the occurrence of breakthrough bleeding in patients using an oral contracep... |
|
|
17-Hydroxysteroid dehydrogenase type 5 gene polymorphism (-71A/G HSD17B5 SNP) and treatment with oral contraceptive pills in PCOS women without metabolic comorbidities.
Gynecol. Endocrinol. 28(8) , 606-10, (2012) We studied (1) the effects of oral contraceptive pills (OCPs) on hirsutism, hormonal and metabolic variables in 49 polycystic ovary syndrome patients without metabolic comorbidities and (2) the effect... |
| (17a)-13-Ethyl-17-hydroxy-18,19-dinorpregna-4,15-dien-20-yn-3-one |
| SH B 331 |
| GESTOGENE |
| (8R,9S,10R,13S,14S,17R)-13-Ethyl-17-ethynyl-17-hydroxy-1,2,6,7,8,9,10,11,12,13,14,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one |
| SH B 33 |
| Femodene |
| EINECS 262-145-8 |
| Gestodene |
| GESTODONE |
| 17a-Ethynyl-13-ethyl-17b-hydroxy-4,15-gonadien-3-one |
| SHG 415 G |
| 17a-Ethynyl-17b-hydroxy-18-methyl-4,15-estradien-3-one |
| Gynovin |
| MFCD00867858 |
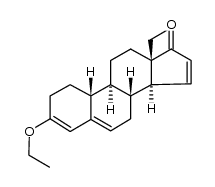 CAS#:1323980-05-7
CAS#:1323980-05-7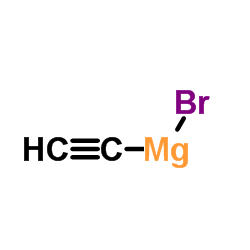 CAS#:4301-14-8
CAS#:4301-14-8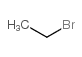 CAS#:74-96-4
CAS#:74-96-4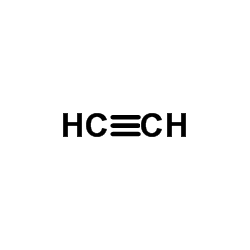 CAS#:74-86-2
CAS#:74-86-2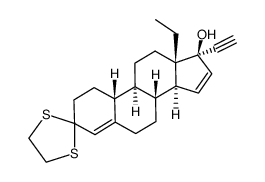 CAS#:74177-02-9
CAS#:74177-02-9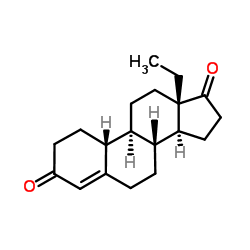 CAS#:21800-83-9
CAS#:21800-83-9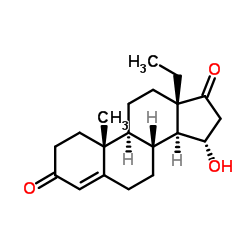 CAS#:60919-46-2
CAS#:60919-46-2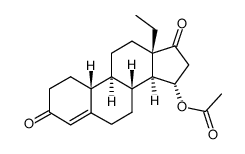 CAS#:104933-97-3
CAS#:104933-97-3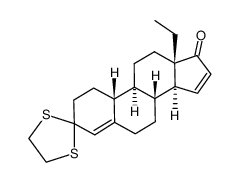 CAS#:74177-01-8
CAS#:74177-01-8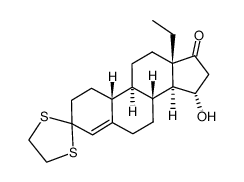 CAS#:74177-00-7
CAS#:74177-00-7
