7,8-Benzoflavone
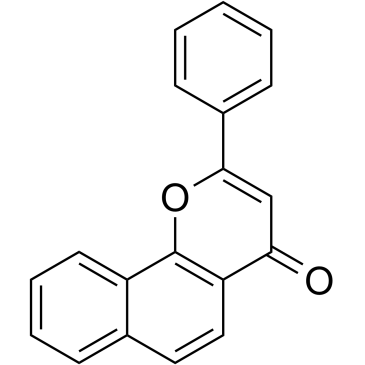
7,8-Benzoflavone structure
|
Common Name | 7,8-Benzoflavone | ||
|---|---|---|---|---|
| CAS Number | 604-59-1 | Molecular Weight | 272.297 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 460.9±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H12O2 | Melting Point | 153-157 °C(lit.) | |
| MSDS | USA | Flash Point | 215.8±22.3 °C | |
Use of 7,8-BenzoflavoneAlpha-Naphthoflavone is a synthetic flavonoid, acts as a potent and competitive aromatase inhibitor with an IC50 and a Ki of 0.5 and 0.2 μM, respectively[1]. |
| Name | α-naphthoflavone |
|---|---|
| Synonym | More Synonyms |
| Description | Alpha-Naphthoflavone is a synthetic flavonoid, acts as a potent and competitive aromatase inhibitor with an IC50 and a Ki of 0.5 and 0.2 μM, respectively[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.5 μM (Aromatase)[1] Ki: 0.2 μM (Aromatase)[1] |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 460.9±45.0 °C at 760 mmHg |
| Melting Point | 153-157 °C(lit.) |
| Molecular Formula | C19H12O2 |
| Molecular Weight | 272.297 |
| Flash Point | 215.8±22.3 °C |
| Exact Mass | 272.083740 |
| PSA | 30.21000 |
| LogP | 4.79 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.695 |
| InChIKey | VFMMPHCGEFXGIP-UHFFFAOYSA-N |
| SMILES | O=c1cc(-c2ccccc2)oc2c1ccc1ccccc12 |
| Storage condition | 2-8°C |
| Stability | Stable. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi,Xn,C,F |
| Risk Phrases | 68-34-11 |
| Safety Phrases | S24/25-S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | QL6250000 |
| HS Code | 2914399090 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 2914399090 |
|---|---|
| Summary | 2914399090. other aromatic ketones without other oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
Development and validation of a liquid-chromatography high-resolution tandem mass spectrometry approach for quantification of nine cytochrome P450 (CYP) model substrate metabolites in an in vitro CYP inhibition cocktail.
Anal. Bioanal. Chem 406(18) , 4453-64, (2014) Knowledge about the cytochrome P450 (CYP) inhibition potential of new drug candidates is important for drug development because of its risk of interactions. For novel psychoactive substances (NPS), co... |
|
|
In vitro cytochrome P450 inhibition potential of methylenedioxy-derived designer drugs studied with a two-cocktail approach.
Arch. Toxicol. 90 , 305-18, (2016) In vitro cytochrome P450 (CYP) inhibition assays are common approaches for testing the inhibition potential of drugs for predicting potential interactions. In contrast to marketed medicaments, drugs o... |
|
|
Cytochrome P450 inhibition potential of new psychoactive substances of the tryptamine class.
Toxicol. Lett. 241 , 82-94, (2015) New psychoactive substances (NPS) are not tested for their cytochrome P450 (CYP) inhibition potential before consumption. Therefore, this potential was explored for tryptamine-derived NPS (TDNPS) incl... |
| Benzo[h]flavone |
| 2-phenylbenzo[h]chromen-4-one |
| 4H-Naphtho[1,2-b]pyran-4-one, 2-phenyl- |
| α-Naphthylflavone |
| 2-Phenyl-4H-benzo[h]chromen-4-one |
| 2-Phenylbenzo(h)chromen-4-one |
| a-Naphthylflavone |
| 2-Phenyl-benzo[h]chromen-4-one |
| alpha-naphthoflavone |
| 7,8-Benzoflavone |
| MFCD00004985 |
| a-Naphthoflavone |
| α-Naphthoflavone |
| 2-Phenyl-4H-naphtho[1,2-b]pyran-4-one |
| EINECS 210-071-1 |
 CAS#:65-85-0
CAS#:65-85-0
