MEK162(ARRY-162,ARRY-438162)
Modify Date: 2024-01-02 12:44:42
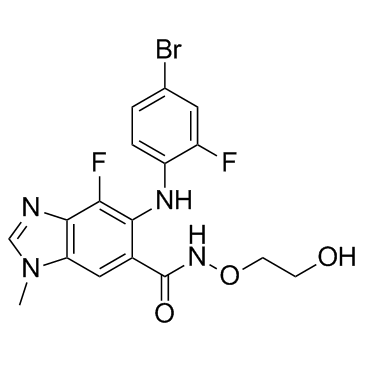
MEK162(ARRY-162,ARRY-438162) structure
|
Common Name | MEK162(ARRY-162,ARRY-438162) | ||
|---|---|---|---|---|
| CAS Number | 606143-89-9 | Molecular Weight | 441.227 | |
| Density | 1.67 | Boiling Point | N/A | |
| Molecular Formula | C17H15BrF2N4O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of MEK162(ARRY-162,ARRY-438162)Binimetinib (MEK162) is an oral and selective MEK1/2 inhibitor with an IC50 of 12 nM. |
| Name | 6-(4-bromo-2-fluoroanilino)-7-fluoro-N-(2-hydroxyethoxy)-3-methylbenzimidazole-5-carboxamide |
|---|---|
| Synonym | More Synonyms |
| Description | Binimetinib (MEK162) is an oral and selective MEK1/2 inhibitor with an IC50 of 12 nM. |
|---|---|
| Related Catalog | |
| Target |
MEK:12 nM (IC50) Autophagy |
| In Vitro | In MCF7 cells, RSK3 or RSK4 expression decreases response to treatment with any of the PI3K inhibitors alone. However, the combination of PI3K inhibition with Binimetinib (MEK162) or BI-D1870 completely reverses the resistance of RSK-expressing cells[2]. Binimetinib (MEK162) blocks basal ERK phosphorylation in all HRAS mutant cell lines. The combination of Everolimus and AZD6244/MEK162 causes a stronger inhibition of S6 kinase than single use of Everolimus on Western blot. The combination of Everolimus and AZD6244/MEK162 also translated in a stronger blockade of cell growth in HRAS mutant cells than single use. Binimetinib (MEK162) shows stronger synergism with Everolimus than AZD6244[3]. |
| In Vivo | Treatment with Binimetinib (ARRY-438162) reduces disease severity in a dose-related manner in both animal models. ARRY-438162 in the CIA model inhibits increases in ankle diameter by 27% and 50% at 1 and 3 mg/kg, while Ibuprofen has 46% inhibition. When combined with Ibuprofen, these same two doses result in 74% and 72% inhibition, respectively. Microscopic examination of the ankle joints show Binimetinib (ARRY-438162) significantly inhibits lesions (inflammation, cartilage damage, pannus formation and bone resorption) by 32% and 60% at 1 and 3 mg/kg, while treatment with Ibuprofen alone results in 17% inhibition, which is not significantly different from the controls. When these two doses of Binimetinib (ARRY-438162) are combined with ibuprofen, the result is 54% and 77% inhibition of joint destruction. In AIA, 3 and 10 mg/kg of Binimetinib (ARRY-438162) inhibit AIA ankle diameter 11% and 34%, while MTX has 33% inhibition. When combined with MTX, 3 and 10 mg/kg of Binimetinib (ARRY-438162) result in 55% and 71% inhibition. Microscopic examination of ankle joints for inflammation and bone resorption also shows improved efficacy versus either compound alone[1]. When Binimetinib (MEK162) is combined with BEZ235, a significant reduction of tumor growth is observed (P=0.01). This increase in antitumor activity is accompanied by a decrease in phospho-ERK and phospho-S6 staining. No significant changes are observed in phospho-4EBP1 staining, a direct target of mTOR activity[2]. |
| Cell Assay | MCF7 cells infected as indicated are seeded in 12-well plates (2×104). After 24 hours, cells are treated with BEZ235 (100 or 200 nM), BKM120 (0.75 or 1 μM), GDC-0941 (1 μM), or MK2206 (2 μM) alone or in combination with Binimetinib (MEK162) (1 μM), BI-D1870 (10 μM), or AZD6244 (1 μM), as indicated in text. Cell numbers are quantified by fixing cells with 4% glutaraldehyde or methanol, washing the cells twice in H2O, and staining the cells with 0.1% crystal violet. The dye is subsequently extracted with 10% acetic acid, and its absorbance is determined (570 nm). Growth curves are performed in triplicate. Viability assays with CellTiter-Glo are performed by plating 2,000 cells in 96-well plates, adding the drug at 24 hours, and assaying 4 to 5 days after drug addition. Cell-cycle and hypodiploid apoptotic cells are quantified by flow cytometry. Briefly, cells are washed with PBS, fixed in cold 70% ethanol, and then stained with propidium iodide while being treated with RNase. Quantitative analysis of sub-G1 cells is carried out in a FACScalibur cytometer using Cell Quest software[2]. |
| Animal Admin | Mice[2] Six-week-old female athymic nude Foxn1nu mice are purchased from Harlan Laboratories. Mice are housed in air-filtered laminar flow cabinets with a 12-hour light/12-hour dark cycle and given food and water ad libitum. Mice are handled with aseptic procedures and allowed to acclimatize to local conditions for 1 week before the experimental manipulations. A 17β-estradiol pellet is implanted subcutaneously into each mouse 1 day before cell injection. 107 MCF-GFP or MCF7-RSK4 cells are resuspended in PBS/Matrigel (1:1) and injected subcutaneously into the right flank of each mouse in 200 μL of final volume. Treatments began when tumors reached an average size of 250 mm3 and are thus considered as established growing xenografts. Mice are treated once daily with placebo, BEZ235, BKM120, MK-2206, or Binimetinib (MEK162) by oral gavage. BEZ235 (25-30 mg/kg, 6IW [6 days on 1 day off]) and BKM120 (30 mg/kg, 6IW) are dissolved in 10% NMP-90% PEG, freshly formulated, and administrated within 30 minutes. MK-2206 (100 mg/kg, 3IW) is formulated in 30% Captisol and Binimetinib (MEK162) (6 mg/kg, BID) in 0.5% Tween-80, 1% carboxymethyl cellulose. For tumor growth studies, mice are treated for 7-24 days, depending on the xenograft model and treatment regime. Tumor xenografts are measured with calipers 3 times a week, and tumor volume is determined using the following formula: (length×width2)×(π/6). At the end of the experiment, the animals are anesthetized with 1.5% isofluorane-air mixture and killed by cervical dislocation. Tumors are removed 2 hours following the last administration. Rats[1] Rat collagen-induced arthritis (CIA) and rat adjuvant-induced arthritis (AIA) models are used to determine efficacy in the subacute inflammation setting. In the CIA studies, rats with established disease, induced by injections of Type II collagen, are treated with 0.3, 1 or 3 mg/kg ARRY-438162 (PO, BID) with or without 30 mg/kg ibuprofen (PO, QD) for six days. Body weight and ankle diameter are used to monitor disease progression on days 0-7. The AIA model is induced by an injection of a lipoidal amine in FCA on day 0. The AIA rats are treated with 1, 3 or 10 mg/kg Binimetinib (ARRY-438162) (PO, QD) beginning on day 8 and continuing for 6 days, with or without the addition of 0.05 mg/kg methotrexate (PO, QD) which is dosed days 0-13. Disease progression is monitored on days 7-14 measuring both paw diameter and body weight. |
| References |
[1]. J Pheneger, et al. 2006, ACR Annual Scientific Meeting. Abst 794. |
| Density | 1.67 |
|---|---|
| Molecular Formula | C17H15BrF2N4O3 |
| Molecular Weight | 441.227 |
| Exact Mass | 440.029541 |
| PSA | 91.90000 |
| LogP | 5.42 |
| Index of Refraction | 1.652 |
| Storage condition | -20℃ |
|
~% 
MEK162(ARRY-162... CAS#:606143-89-9 |
| Literature: NOVARTIS AG; ARRAY BIOPHARMA INC.; KRELL, Christoph, Max; MISUN, Marian; NIEDERER, Daniel, Andreas; PACHINGER, Werner, Heinz; WOLF, Marie-christine; ZIMMERMANN, Daniel; LIU, Weidong; STENGEL, Peter, J.; NICHOLS, Paul Patent: WO2014/63024 A1, 2014 ; |
|
~% 
MEK162(ARRY-162... CAS#:606143-89-9 |
| Literature: NOVARTIS AG; ARRAY BIOPHARMA INC.; KRELL, Christoph, Max; MISUN, Marian; NIEDERER, Daniel, Andreas; PACHINGER, Werner, Heinz; WOLF, Marie-christine; ZIMMERMANN, Daniel; LIU, Weidong; STENGEL, Peter, J.; NICHOLS, Paul Patent: WO2014/63024 A1, 2014 ; |
|
~% 
MEK162(ARRY-162... CAS#:606143-89-9 |
| Literature: NOVARTIS PHARMA AG; AMGEN INC.; HUANG, Xizhong; PETERS, Malte; SCHUMACHER, Karl, Maria; CAO, Zhu, Alexander; GANSERT, Jennifer, Lorraine; CHANG, David, Dong, Eun; BELTRAN, Pedro Patent: WO2013/142182 A2, 2013 ; |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| ARRY-438162 |
| 1H-Benzimidazole-6-carboxamide, 5-[(4-bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl- |
| Binimetinib |
| 6-(4-bromo-2-fluorophenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2-hydroxyethoxy)amide |
| MEK-162 |
| 6-(4-bromo-2-fluorophenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid-(2-hydroxyethyoxy)amide |
| 5-[(4-Bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzimidazole-6-carboxamide |
| ARRY-162 |
| MEK162 |
| QCR-138 |
| cc-455 |
![Methyl 5-amino-4-fluoro-1-methyl-1H-benzo[d]imidazole-6-carboxylate structure](https://www.chemsrc.com/caspic/299/918321-20-7.png)