D-Saccharic acid 1,4-lactone hydrate
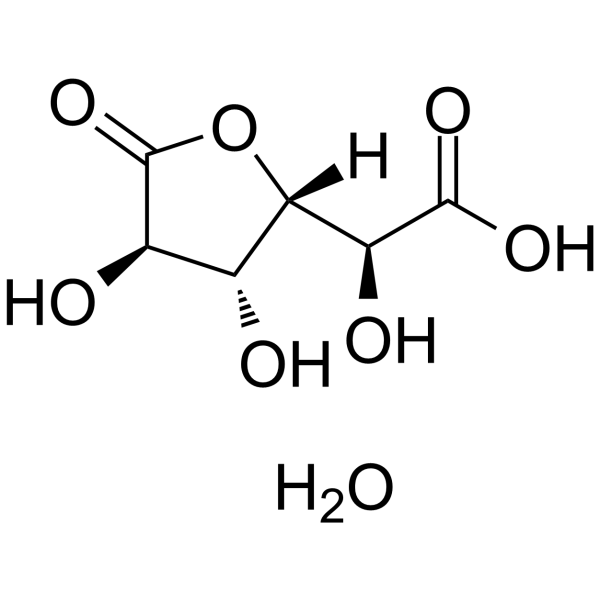
D-Saccharic acid 1,4-lactone hydrate structure
|
Common Name | D-Saccharic acid 1,4-lactone hydrate | ||
|---|---|---|---|---|
| CAS Number | 61278-30-6 | Molecular Weight | 192.12400 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C6H10O8 | Melting Point | 80-82ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
Use of D-Saccharic acid 1,4-lactone hydrateD-Saccharic acid 1,4-lactone hydrate is a potent β-glucuronidase inhibitor (IC50=48.4 μM). D-Saccharic acid 1,4-lactone hydrate can be used as a standard drug compared with novel β-glucuronidase inhibitors. D-Saccharic acid 1,4-lactone hydrate possesses anticarcinogenic, detoxifying, and antioxidant properties[1][2]. |
| Name | (2S)-2-[(3R,4R)-3,4-dihydroxy-5-oxooxolan-2-yl]-2-hydroxyacetic acid,hydrate |
|---|---|
| Synonym | More Synonyms |
| Description | D-Saccharic acid 1,4-lactone hydrate is a potent β-glucuronidase inhibitor (IC50=48.4 μM). D-Saccharic acid 1,4-lactone hydrate can be used as a standard drug compared with novel β-glucuronidase inhibitors. D-Saccharic acid 1,4-lactone hydrate possesses anticarcinogenic, detoxifying, and antioxidant properties[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Melting Point | 80-82ºC |
|---|---|
| Molecular Formula | C6H10O8 |
| Molecular Weight | 192.12400 |
| Exact Mass | 192.02700 |
| PSA | 124.29000 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| HS Code | 2932190090 |
| HS Code | 2932190090 |
|---|---|
| Summary | 2932190090 other compounds containing an unfused furan ring (whether or not hydrogenated) in the structure VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |
|
Phase II metabolism in human skin: skin explants show full coverage for glucuronidation, sulfation, N-acetylation, catechol methylation, and glutathione conjugation.
Drug Metab. Dispos. 43(1) , 126-39, (2014) Although skin is the largest organ of the human body, cutaneous drug metabolism is often overlooked, and existing experimental models are insufficiently validated. This proof-of-concept study investig... |
|
|
A High Throughput Assay for Discovery of Bacterial β-Glucuronidase Inhibitors.
Curr. Chem. Genomics 5 , 13-20, (2011) CPT-11 is a widely-used anti-cancer drug that is converted in vivo to its active metabolite, SN-38. In the liver, enzymes detoxify SN-38 by coupling it to a glucuronidate moiety and this inactive comp... |
|
|
Characterization of an MPS I-H knock-in mouse that carries a nonsense mutation analogous to the human IDUA-W402X mutation.
Mol. Genet. Metab. 99(1) , 62-71, (2010) Here we report the characterization of a knock-in mouse model for the autosomal recessive disorder mucopolysaccharidosis type I-Hurler (MPS I-H), also known as Hurler syndrome. MPS I-H is the most sev... |
| D-Saccharolactone |
| D-Saccharic acid 1,4-lactone monohydrate |
| D-Glucaro-1,4-lactone Monohydrate |
| 1,4-Lactone-D-glucaric Acid Monohydrate |
| D-Glucaro-1,4-lacton |
| D-Glucaric acid-1,4-lactone |
| MFCD00149334 |