Oxalic acid dihydrate
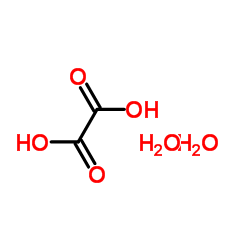
Oxalic acid dihydrate structure
|
Common Name | Oxalic acid dihydrate | ||
|---|---|---|---|---|
| CAS Number | 6153-56-6 | Molecular Weight | 126.065 | |
| Density | 1,65 g/cm3 | Boiling Point | 108-109°C | |
| Molecular Formula | C2H6O6 | Melting Point | 104-106 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 157°C | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
| Name | Oxalic acid dihydrate |
|---|---|
| Synonym | More Synonyms |
| Density | 1,65 g/cm3 |
|---|---|
| Boiling Point | 108-109°C |
| Melting Point | 104-106 °C(lit.) |
| Molecular Formula | C2H6O6 |
| Molecular Weight | 126.065 |
| Flash Point | 157°C |
| Exact Mass | 126.016441 |
| PSA | 93.06000 |
| Vapour density | 4.4 (vs air) |
| Vapour Pressure | <0.01 mm Hg ( 20 °C) |
| Storage condition | Store at RT. |
| Water Solubility | 138 g/L (20 ºC) |
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302 + H312-H318 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | R21/22 |
| Safety Phrases | S24/25 |
| RIDADR | UN 3261 8/PG 3 |
| WGK Germany | 1 |
| RTECS | RO2450000 |
| Packaging Group | III |
| Hazard Class | 8 |
| HS Code | 29171100 |
|
~55% 
Oxalic acid dih... CAS#:6153-56-6 |
| Literature: Zaher, Joseph J.; Fritzler, Bryan C.; Hutchison, Scott N. Patent: US2007/66847 A1, 2007 ; Location in patent: Page/Page column 2-3 ; |
| Precursor 1 | |
|---|---|
| DownStream 10 | |
| HS Code | 2917119000 |
|---|---|
| Summary | 2917119000 oxalic acid salts and esters VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Lefetamine, a controlled drug and pharmaceutical lead of new designer drugs: synthesis, metabolism, and detectability in urine and human liver preparations using GC-MS, LC-MS(n), and LC-high resolution-MS/MS.
Anal. Bioanal. Chem 407(6) , 1545-57, (2015) Lefetamine (N,N-dimethyl-1,2-diphenylethylamine, L-SPA) was marketed as an opioid analgesic in Japan and Italy. After being widely abused, it became a controlled substance. It seems to be a pharmaceut... |
|
|
An Assessment of Engineered Calcium Oxalate Crystal Formation on Plant Growth and Development as a Step toward Evaluating Its Use to Enhance Plant Defense.
PLoS ONE 10 , e0141982, (2015) The establishment of new approaches to control chewing insects has been sought not only for direct use in reducing crop loss but also in managing resistance to the pesticides already in use. Engineere... |
|
|
Thermodynamics for complex formation between palladium(ii) and oxalate.
Dalton Trans. 43(32) , 12243-50, (2014) Complex formation between [Pd(H2O)4](2+) and oxalate (ox = C2O4(2-)) has been studied spectrophoto-metrically in aqueous solution at variable temperature, ionic strength and pH. Thermodynamic paramete... |
| MFCD00149102 |
| oxalic acid,dihydrate |
| EINECS 205-634-3 |
 CAS#:494-38-2
CAS#:494-38-2 CAS#:82207-69-0
CAS#:82207-69-0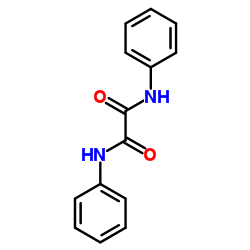 CAS#:620-81-5
CAS#:620-81-5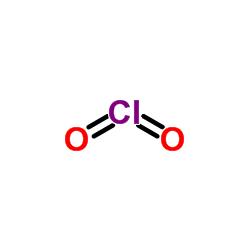 CAS#:10049-04-4
CAS#:10049-04-4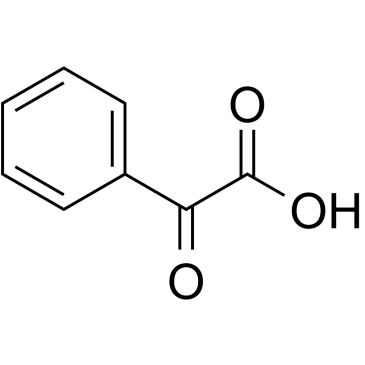 CAS#:611-73-4
CAS#:611-73-4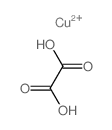 CAS#:814-91-5
CAS#:814-91-5 CAS#:6309-61-1
CAS#:6309-61-1 CAS#:640-67-5
CAS#:640-67-5 CAS#:6047-25-2
CAS#:6047-25-2 CAS#:100516-90-3
CAS#:100516-90-3
