(3β,7α)-Cholest-5-ene-3,7,25-triol
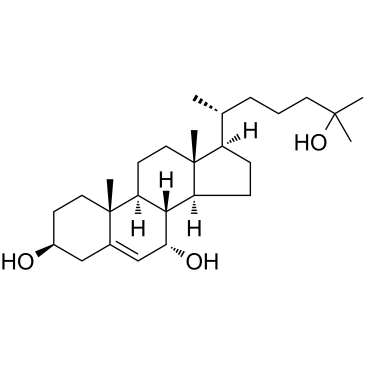
(3β,7α)-Cholest-5-ene-3,7,25-triol structure
|
Common Name | (3β,7α)-Cholest-5-ene-3,7,25-triol | ||
|---|---|---|---|---|
| CAS Number | 64907-22-8 | Molecular Weight | 418.652 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 548.5±35.0 °C at 760 mmHg | |
| Molecular Formula | C27H46O3 | Melting Point | 235 - 236 °C | |
| MSDS | Chinese USA | Flash Point | 230.5±20.5 °C | |
Use of (3β,7α)-Cholest-5-ene-3,7,25-triol7α, 25-dihydroxycholesterol (7α,25-OHC) is a potent and selective agonist and endogenous ligand of the orphan GPCR receptor EBI2 (GPR183). 7α, 25-dihydroxycholesterol is highly potent at activating EBI2 (EC50=140 pM; Kd=450 pM). 7α, 25-dihydroxycholesterol can serve as a chemokine directing migration of B cells, T cells and dendritic cells[1][2]. |
| Name | 7α,25-Dihydroxycholesterol |
|---|---|
| Synonym | More Synonyms |
| Description | 7α, 25-dihydroxycholesterol (7α,25-OHC) is a potent and selective agonist and endogenous ligand of the orphan GPCR receptor EBI2 (GPR183). 7α, 25-dihydroxycholesterol is highly potent at activating EBI2 (EC50=140 pM; Kd=450 pM). 7α, 25-dihydroxycholesterol can serve as a chemokine directing migration of B cells, T cells and dendritic cells[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | In vitro, 7α, 25-dihydroxycholesterol (7α,25-OHC) stimulates the migration of EBI2-expressing mouse B and T cells with half-maximum effective concentration values around 500 pM, but had no effect on EBI2-deficient cells[1]. |
| In Vivo | EBI2-deficient B cells or normal B cells desensitized by 7α,25-Dihydroxycholesterol (1 μM; 1.5 hours) pre-treatment shows reduced homing to follicular areas of the spleen[1]. |
| References |
[1]. Liu C, et al. Oxysterols direct B-cell migration through EBI2. Nature. 2011 Jul 27;475(7357):519-23. |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 548.5±35.0 °C at 760 mmHg |
| Melting Point | 235 - 236 °C |
| Molecular Formula | C27H46O3 |
| Molecular Weight | 418.652 |
| Flash Point | 230.5±20.5 °C |
| Exact Mass | 418.344696 |
| PSA | 60.69000 |
| LogP | 5.75 |
| Vapour Pressure | 0.0±3.3 mmHg at 25°C |
| Index of Refraction | 1.550 |
| InChIKey | BQMSKLCEWBSPPY-IKVTXIKFSA-N |
| SMILES | CC(CCCC(C)(C)O)C1CCC2C3C(O)C=C4CC(O)CCC4(C)C3CCC12C |
| RIDADR | NONH for all modes of transport |
|---|
|
Detection of dihydroxycholesterols in human plasma using HPLC-ESI-MS/MS.
Steroids 99 , 131-8, (2015) We report a straightforward sample preparation procedure and a direct liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) method for the analysis of 7alpha,25-dihydro... |
|
|
Studies on the relationships between 7 alpha-hydroxylation and the ability of 25- and 27-hydroxycholesterol to suppress the activity of HMG-CoA reductase.
Biochim. Biophys. Acta 1344(3) , 241-9, (1997) The metabolism of 25-hydroxycholesterol in different cell types was studied and the role of 7 alpha-hydroxylation for the effect of 25-hydroxycholesterol on the activity of HMG-CoA reductase was deter... |
|
|
Molecular characterization of oxysterol binding to the Epstein-Barr virus-induced gene 2 (GPR183).
J. Biol. Chem. 287(42) , 35470-83, (2012) Oxysterols are oxygenated cholesterol derivates that are emerging as a physiologically important group of molecules. Although they regulate a range of cellular processes, only few oxysterol-binding ef... |
| Cholest-5-ene-3,7,25-triol, (3β,7α)- |
| 7.α.,25-dihydroxy Cholesterol |
| (3β,7α)-Cholest-5-ene-3,7,25-triol |