Avermectin B1a
Modify Date: 2024-01-02 17:27:56
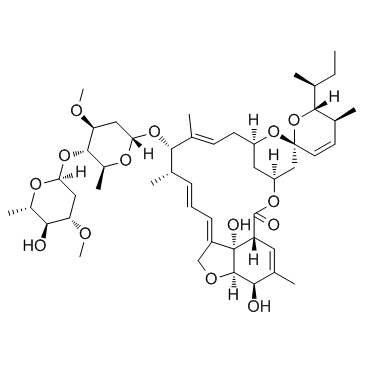
Avermectin B1a structure
|
Common Name | Avermectin B1a | ||
|---|---|---|---|---|
| CAS Number | 65195-55-3 | Molecular Weight | 873.077 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 940.9±65.0 °C at 760 mmHg | |
| Molecular Formula | C48H72O14 | Melting Point | 157-162ºC | |
| MSDS | N/A | Flash Point | 268.1±27.8 °C | |
Use of Avermectin B1aAvermectin B1a is an antiparasitic agent that paralyzes nematodes without causing hypercontraction or flaccid paralysis. |
| Name | avermectin B1a |
|---|---|
| Synonym | More Synonyms |
| Description | Avermectin B1a is an antiparasitic agent that paralyzes nematodes without causing hypercontraction or flaccid paralysis. |
|---|---|
| Related Catalog | |
| In Vitro | [3H]AVM B1a preferentially binds to synaptic membranes from several regions of rat brain. [3H]AVM B1a specific binding to intact monolayers of granule cells increases rapidly with time of incubation and reaches equilibrium after approximately 20 min at 24°C. Higher concentrations of [3H]AVM B1a leads to markedly greater nonspecific binding, 60% at 25 nM. Various AVM analogs also produce concentration-dependent inhibition of [3H]AVM B1a binding in intact cerebellar neurons. AVM B1a and moxidectin are similar in potency (IC50 values, 120 and 126 nM, respectively)[3]. AVMB1a-stimulated chloride efflux from mouse brain synaptic vesicles results from the activation of GABA-insensitive chloride channels and that this action is distinct from their previously documented effects on GABA-gated chloride channels in mouse brain preparations[4]. |
| In Vivo | Bacteria are significantly inhibited when the AVM B1a concentration is higher than 83.3 mg/kg, while fungi are less impaired in soil. Soil respiration is also inhibited by high concentration AVM B1a, which differs with soil types. The half lethal dosage (LD50) of AVM B1a to soil earthworm is estimated as 4.63 mg × cm2 in filter paper contact test, and as 24.13 and 17.06 mg/kg, respectively after treated 7 and 14 days in artificial soil[1]. Iin artificial soil, the LC50 of AVM B1a on earthworms are 24.1 mg/kg and 17.1 mg/kg, respectively, for 7 and 14 days. About 80.0% and 94.8% of the accumulated AVM B1a are eliminated respectively in two groups within 1 day after they are exposed to AVM B1a-free soil, but a trace amount of AVM B1a is found for a relative long time in earthworms[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 940.9±65.0 °C at 760 mmHg |
| Melting Point | 157-162ºC |
| Molecular Formula | C48H72O14 |
| Molecular Weight | 873.077 |
| Flash Point | 268.1±27.8 °C |
| Exact Mass | 872.492188 |
| PSA | 170.06000 |
| LogP | 6.51 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.571 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | UN 2588 |
|---|---|
| Packaging Group | II |
| Hazard Class | 6.1(a) |
| HS Code | 2932999099 |
| Precursor 9 | |
|---|---|
| DownStream 2 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| 5-O-Demethyl Antibiotic C 076A1a |
| Abamectinum [Latin] |
| Abamectin B1a |
| AVERMECTIN B1A |
| (1'R,2S,4'S,5S,6R,8'R,10'E,12'S,13'S,14'E,16'E,20'R,21'R,24'S)-6-[(2S)-Butan-2-yl]-21',24'-dihydroxy-5,11',13',22'-tetramethyl-2'-oxo-5,6-dihydrospiro[pyran-2,6'-[3,7,19]trioxatetracyclo[15.6.1.1.0]pentacosa[10,14,16,22]tetraen]-12'-yl 2,6-dideoxy-4-O-(2,6-dideoxy-3-O-methyl-α-L-arabino-hexopyranosyl)-3-O-methyl-α-L-arabino-hexopyranoside |
| Abamectine [French] |
| [3H]-Abamectin B1a |
| (1'R,2S,4'S,5S,6R,8'R,10'E,12'S,13'S,14'E,16'E,20'R,21'R,24'S)-6-[(2S)-2-Butanyl]-21',24'-dihydroxy-12'-{[(2R,4S,5S,6S)-5-{[(2S,4S,5S,6S)-5-hydroxy-4-methoxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-4-methoxy-6-methyltetrahydro-2H-pyran-2-yl]oxy}-5,11',13',22'-tetramethyl-5,6-dihydro-2'H-spiro[pyran-2,6'-[3,7,19]trioxatetracyclo[15.6.1.1.0]pentacosa[10,14,16,22]tetraen]-2'-one |
| (1'R,2S,4'S,5S,6R,8'R,10'E,12'S,13'S,14'E,16'E,20'R,21'R,24'S)-6-[(2S)-2-Butanyl]-21',24'-dihydroxy-5,11',13',22'-tetramethyl-2'-oxo-5,6-dihydrospiro[pyran-2,6'-[3,7,19]trioxatetracyclo[15.6.1.1.0]pentacosa[10,14,16,22]tetraen]-12'-yl 2,6-dideoxy-4-O-(2,6-dideoxy-3-O-methyl-α-L-arabino-hexopyranosyl)-3-O-methyl-α-L-arabino-hexopyranoside |
| Abamectin komponente B1a |
| 22,23-dihydroavermectinh B1a |
| abamectin component B1a |
| Avermectin B(1)a |
| Abamectina [Spanish] |
| (2aE,4E,5'S,6S,6'R,7S,8E,11R,13S,15S,17aR,20R,20aR,20bS)-6'-[(2S)-butan-2-yl]-20,20b-dihydroxy-5',6,8,19-tetramethyl-17-oxo-5',6,6',10,11,14,15,17,17a,20,20a,20b-dodecahydro-2H,7H-spiro[11,15-methanofuro[4,3,2-pq][2,6]benzodioxacyclooctadecine-13,2'-pyran]-7-yl 2,6-dideoxy-4-O-(2,6-dideoxy-3-O-methyl-α-L-arabino-hexopyranosyl)-3-O-methyl-α-L-arabino-hexopyranoside |
| (2aE,4E,8E)-(5'S,6S,6'R,7S,11R,13S,15S,17aR,20R,20aR,20bS)-6'-((S)-sec-Butyl)-5',6,6',7,10,11,14,15,17a,20,20a,20b-dodecahydro-20,20b-dihydroxy-5',6,8,19-tetramethyl-17-oxospiro(11,15-methano-2H,13H,17H-furo(4,3,2-pq)(2,6)benzodioxacyclooctadecin-13,2'-(2H)pyran)-7-yl 2,6-dideoxy-4-O-(2,6-dideoxy-3-O-methyl-α-L-arabino-hexopyranosyl)-3-O-methyl-α-L-arabino-hexopyranoside |
| Vertimec(R)018SC |
| Caswell No. 063AB |
| (1'R,2S,4'S,5S,6R,8'R,10'E,12'S,13'S,14'E,16'E,20'R,21'R,24'S)-6-[(2S)-2-Butanyl]-21',24'-dihydroxy-5,11',13',22'-tetramethyl-2'-oxo-5,6-dihydrospiro[pyran-2,6'-[3,7,19]trioxatetracyclo[15.6.1.1. 0]pentacosa[10,14,16,22]tetraen]-12'-yl 2,6-dideoxy-4-O-(2,6-dideoxy-3-O-methyl-α-L-arabino-hexopyranosyl)-3-O-methyl-α-L-arabino-hexopyranoside |
| (1'R,2S,4'S,5S,6R,8'R,10'E,12'S,13'S,14'E,16'E,20'R,21'R,24'S)-6-[(2S)-2-Butanyl]-21',24'-dihydroxy-5,11',13',22'-tetramethyl-2'-oxo-5,6-dihydrospiro[pyran-2,6'-[3,7,19]trioxatetracyclo[15.6.1.1.0]pentacosa[10,14,16,22]tetraen]-12'-yl-2,6-dideoxy-4-O-(2,6-dideoxy-3-O-methyl-α-L-arabino-hexopyranosyl)-3-O-methyl-α-L-arabino-hexopyranoside |
| Antibiotic C 076B1a |
 CAS#:81924-42-7
CAS#:81924-42-7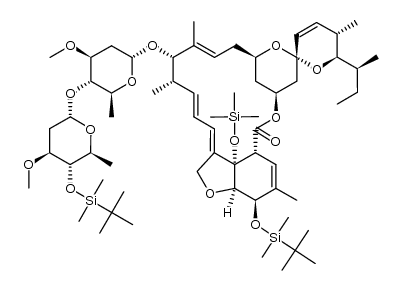 CAS#:106544-70-1
CAS#:106544-70-1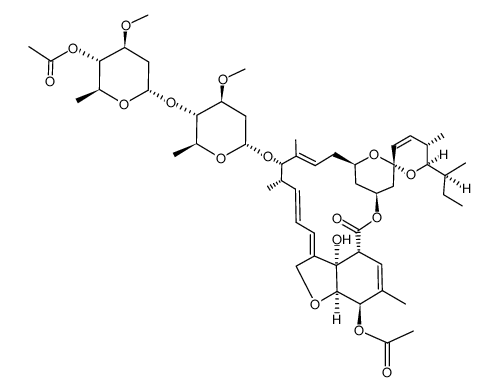 CAS#:71826-88-5
CAS#:71826-88-5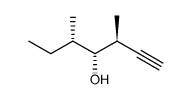 CAS#:87319-06-0
CAS#:87319-06-0 CAS#:87319-05-9
CAS#:87319-05-9 CAS#:87319-07-1
CAS#:87319-07-1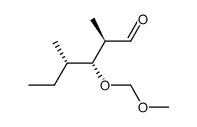 CAS#:87319-04-8
CAS#:87319-04-8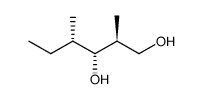 CAS#:78122-99-3
CAS#:78122-99-3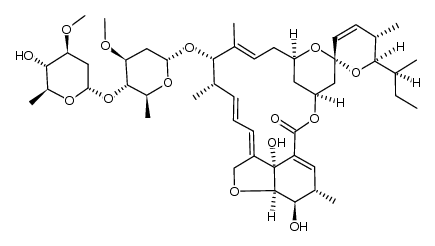 CAS#:110415-68-4
CAS#:110415-68-4