1,10-Phenanthroline
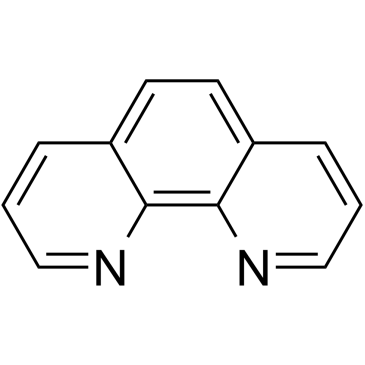
1,10-Phenanthroline structure
|
Common Name | 1,10-Phenanthroline | ||
|---|---|---|---|---|
| CAS Number | 66-71-7 | Molecular Weight | 180.205 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 365.1±15.0 °C at 760 mmHg | |
| Molecular Formula | C12H8N2 | Melting Point | 114-117 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 164.8±11.7 °C | |
| Symbol |


GHS06, GHS09 |
Signal Word | Danger | |
Use of 1,10-Phenanthrolineo-Phenanthroline (1,10-Phenanthroline), a metal chelator, prevents the induction of chromosomal aberrations in streptozotocin-treated cells. o-Phenanthroline (1,10-Phenanthroline) forms a red chelate with Fe2+ that absorbs maximally at 510 nm[1]. |
| Name | 1,10-phenanthroline |
|---|---|
| Synonym | More Synonyms |
| Description | o-Phenanthroline (1,10-Phenanthroline), a metal chelator, prevents the induction of chromosomal aberrations in streptozotocin-treated cells. o-Phenanthroline (1,10-Phenanthroline) forms a red chelate with Fe2+ that absorbs maximally at 510 nm[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 365.1±15.0 °C at 760 mmHg |
| Melting Point | 114-117 °C(lit.) |
| Molecular Formula | C12H8N2 |
| Molecular Weight | 180.205 |
| Flash Point | 164.8±11.7 °C |
| Exact Mass | 180.068741 |
| PSA | 25.78000 |
| LogP | 1.78 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.740 |
| InChIKey | DGEZNRSVGBDHLK-UHFFFAOYSA-N |
| SMILES | c1cnc2c(c1)ccc1cccnc12 |
| Storage condition | Refrigerator |
| Stability | Stable. Hygroscopic. Store under nitrogen. Incompatible with strong acids, strong oxidizing agents. |
| Water Solubility | slightly soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |


GHS06, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H410 |
| Precautionary Statements | P273-P301 + P310-P501 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R25;R50/53 |
| Safety Phrases | S45-S60-S61 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | SF8437000 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 29339990 |
| Precursor 7 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Comprehensive analysis of heterotrimeric G-protein complex diversity and their interactions with GPCRs in solution.
Proc. Natl. Acad. Sci. U. S. A. 112(11) , E1181-90, (2015) Agonist binding to G-protein-coupled receptors (GPCRs) triggers signal transduction cascades involving heterotrimeric G proteins as key players. A major obstacle for drug design is the limited knowled... |
|
|
Essential Oil from Clove Bud (Eugenia aromatica Kuntze) Inhibit Key Enzymes Relevant to the Management of Type-2 Diabetes and Some Pro-oxidant Induced Lipid Peroxidation in Rats Pancreas in vitro.
J. Oleo Sci. 64 , 775-82, (2015) The inhibition of enzymes involved in the breakdown of carbohydrates is considered a therapeutic approach to the management of type-2 diabetes. This study sought to investigate the effects of essentia... |
|
|
Monitoring the inorganic chemical reaction by surface-enhanced Raman spectroscopy: A case of Fe³⁺ to Fe²⁺ conversion.
Talanta 146 , 452-6, (2015) Monitoring the process of organic chemical reactions to study the kinetics by surface-enhanced Raman spectroscopy (SERS) is currently of immense interest. However, monitoring the inorganic chemical re... |
| 1,10-Phenanthroline |
| 4,5-Diazaphenanthrene |
| 4,5-Phenanthroline |
| ORTHOPHENANTHROLINE |
| EINECS 200-629-2 |
| 1,10-o-Phenanthroline |
| o-Phenanthroline |
| β-Phenanthroline |
| 1,10-Fenanthroline |
| phenanthroline |
| MFCD00011678 |
| 1,10-Fenanthrolin |
| phen |
| 10-Phenanthroline |
| T B666 CN NNJ |
 CAS#:150-90-3
CAS#:150-90-3 CAS#:3188-84-9
CAS#:3188-84-9 CAS#:65115-91-5
CAS#:65115-91-5 CAS#:594-27-4
CAS#:594-27-4 CAS#:815-68-9
CAS#:815-68-9 CAS#:158753-17-4
CAS#:158753-17-4![(8R,9S,10R,13S,14S,17S)-3-butoxy-10,13,17-trimethyl-1,2,7,8,9,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-17-ol Structure](https://image.chemsrc.com/caspic/114/2143-61-5.png) CAS#:2143-61-5
CAS#:2143-61-5 CAS#:109559-47-9
CAS#:109559-47-9 CAS#:1082-19-5
CAS#:1082-19-5 CAS#:40311-13-5
CAS#:40311-13-5 CAS#:42906-19-4
CAS#:42906-19-4 CAS#:54258-41-2
CAS#:54258-41-2 CAS#:40000-20-2
CAS#:40000-20-2 CAS#:14768-11-7
CAS#:14768-11-7 CAS#:14783-55-2
CAS#:14783-55-2 CAS#:142942-21-0
CAS#:142942-21-0![2-(4-methoxyphenyl)-9-[6-[9-(4-methoxyphenyl)-1,10-phenanthrolin-2-yl]hexyl]-1,10-phenanthroline structure](https://image.chemsrc.com/caspic/429/142942-24-3.png) CAS#:142942-24-3
CAS#:142942-24-3
