Cholestenone-13C
Modify Date: 2025-08-25 11:43:05
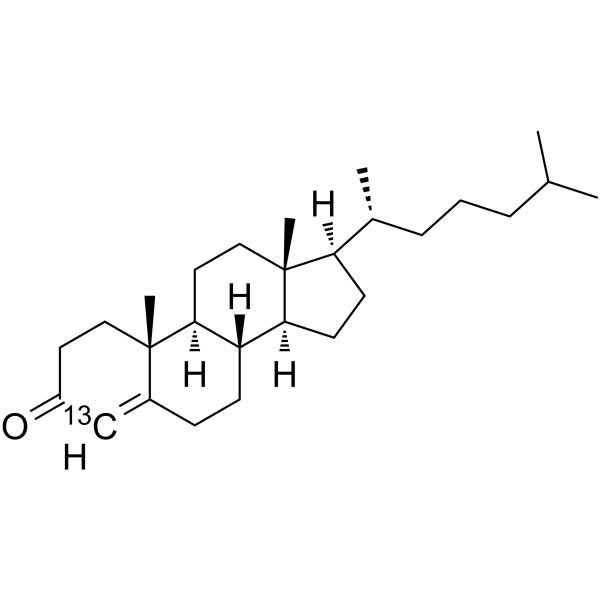
Cholestenone-13C structure
|
Common Name | Cholestenone-13C | ||
|---|---|---|---|---|
| CAS Number | 68165-57-1 | Molecular Weight | 385.63 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C2613CH44O | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Cholestenone-13CCholestenone-13C is the 13C labeled Cholestenone. Cholestenone (4-Cholesten-3-one), the intermediate oxidation product of cholesterol, is metabolized primarily in the liver. Cholestenone is highly mobile in membranes and influences cholesterol flip-flop and efflux. Cholestenone may cause long-term functional defects in cells[1][2][3]. |
| Name | 4-cholesten-3-one-4-13c |
|---|---|
| Synonym | More Synonyms |
| Description | Cholestenone-13C is the 13C labeled Cholestenone. Cholestenone (4-Cholesten-3-one), the intermediate oxidation product of cholesterol, is metabolized primarily in the liver. Cholestenone is highly mobile in membranes and influences cholesterol flip-flop and efflux. Cholestenone may cause long-term functional defects in cells[1][2][3]. |
|---|---|
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Molecular Formula | C2613CH44O |
|---|---|
| Molecular Weight | 385.63 |
| Exact Mass | 385.34300 |
| PSA | 17.07000 |
| LogP | 7.59690 |
| <4-13C>-Cholest-4-en-3-on |
| [4-13C]cholest-4-en-3-one |
| <4.4.2>Propelle-2,4,8,11-tetraen |
| 1,4-dihydro-4a,8a-ethenonaphthalene |
| <4.4.2>Propella 2,4,8,11-tetraen |