3-nitro-2-pyridinesulfenyl chloride
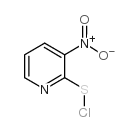
3-nitro-2-pyridinesulfenyl chloride structure
|
Common Name | 3-nitro-2-pyridinesulfenyl chloride | ||
|---|---|---|---|---|
| CAS Number | 68206-45-1 | Molecular Weight | 190.60800 | |
| Density | 1.58g/cm3 | Boiling Point | 376.8ºC at 760mmHg | |
| Molecular Formula | C5H3ClN2O2S | Melting Point | 205ºC (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 181.7ºC | |
| Symbol |

GHS05 |
Signal Word | Danger | |
| Name | (3-nitropyridin-2-yl) thiohypochlorite |
|---|---|
| Synonym | More Synonyms |
| Density | 1.58g/cm3 |
|---|---|
| Boiling Point | 376.8ºC at 760mmHg |
| Melting Point | 205ºC (dec.)(lit.) |
| Molecular Formula | C5H3ClN2O2S |
| Molecular Weight | 190.60800 |
| Flash Point | 181.7ºC |
| Exact Mass | 189.96000 |
| PSA | 84.01000 |
| LogP | 2.75890 |
| InChIKey | WTKQMHWYSBWUBE-UHFFFAOYSA-N |
| SMILES | O=[N+]([O-])c1cccnc1SCl |
| Storage condition | 2-8°C |
| Water Solubility | dichloromethane: soluble(lit.) |
| Symbol |

GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H314 |
| Precautionary Statements | P280-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | C |
| Risk Phrases | R34 |
| Safety Phrases | 26-27-36/37/39-45 |
| RIDADR | UN 3261 8/PG 2 |
| WGK Germany | 3 |
|
Synthesis of disulfide-bridged fragments of omega-conotoxins GVIA and MVIIA. Use of Npys as a protecting/activating group for cysteine in Fmoc syntheses.
Int. J. Pept. Protein Res. 43(4) , 363-6, (1994) The 3-nitro-2-pyridinesulphenyl (Npys) moiety is finding increasing utility as a protecting-activating group for cysteine, particularly in the synthesis of cyclic and unsymmetrical disulfides using th... |
|
|
Synthesis and stability of 3-nitro-2-pyridinesulfenyl chloride (NpysCl).
Int. J. Pept. Protein Res. 42(2) , 159-64, (1993) 3-Nitro-2-pyridinesulfenyl chloride (NpysCl) is the starting material for the synthesis of N-, O- and S-Npys-protected amino acids. Two efficient, novel synthetic routes to NpysCl are described. The s... |
|
|
Discriminative affinity labelling of opioid receptors by enkephalin and morphiceptin analogues containing 3-nitro-2-pyridinesulphenyl-activated thiol residues.
J. Chromatogr. A. 597(1-2) , 425-8, (1992) The thiol groups of leucinthiol, cysteamine and cysteine incorporated into opioid peptides enkephalin and morphiceptin were activated by the 3-nitro-2-pyridinesulphenyl (Npys) group to form mixed disu... |
| 3-Nitro-2-pyridinesulfenyl chloride |
| 3-nitropyridine-2-sulfenyl chloride |
| 3-nitro-2-pyridinylsulfenyl chloride |
| Pyridine,2-chlorothio-3-nitro |
| 2-Pyridinesulfenyl chloride,3-nitro |
| 3-nitropyridinesulfenyl chloride |
| 3-nitro-pyridin-2-ylsulfenyl chloride |
| Npys-Cl |
| Npys |
| MFCD00274609 |
| 3-Nitro-2-pyridinesulfenyl |
 CAS#:69212-31-3
CAS#:69212-31-3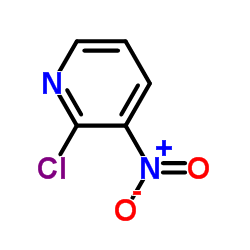 CAS#:5470-18-8
CAS#:5470-18-8![2-[(4-methoxyphenyl)methylsulfanyl]-3-nitropyridine Structure](https://image.chemsrc.com/caspic/413/153815-22-6.png) CAS#:153815-22-6
CAS#:153815-22-6 CAS#:76880-29-0
CAS#:76880-29-0