(E)-4,4,4-TRIFLUORO-1-PHENYL-BUT-2-EN-1-ONE
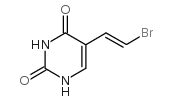
(E)-4,4,4-TRIFLUORO-1-PHENYL-BUT-2-EN-1-ONE structure
|
Common Name | (E)-4,4,4-TRIFLUORO-1-PHENYL-BUT-2-EN-1-ONE | ||
|---|---|---|---|---|
| CAS Number | 69304-49-0 | Molecular Weight | 217.02000 | |
| Density | 1.881g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C6H5BrN2O2 | Melting Point | 189-194ºC (dec.)(lit.) | |
| MSDS | USA | Flash Point | N/A | |
| Name | 5-[(E)-2-bromoethenyl]-1H-pyrimidine-2,4-dione |
|---|---|
| Synonym | More Synonyms |
| Density | 1.881g/cm3 |
|---|---|
| Melting Point | 189-194ºC (dec.)(lit.) |
| Molecular Formula | C6H5BrN2O2 |
| Molecular Weight | 217.02000 |
| Exact Mass | 215.95300 |
| PSA | 65.72000 |
| LogP | 0.42880 |
| Index of Refraction | 1.685 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | UV9020500 |
|
~86% 
(E)-4,4,4-TRIFL... CAS#:69304-49-0 |
| Literature: Rega Instituut VZW; University of Birmingham Patent: US4424211 A1, 1984 ; |
|
~82% 
(E)-4,4,4-TRIFL... CAS#:69304-49-0 |
| Literature: Eger; Jalalian; Schmidt Journal of Heterocyclic Chemistry, 1995 , vol. 32, # 1 p. 211 - 218 |
|
~% 
(E)-4,4,4-TRIFL... CAS#:69304-49-0 |
| Literature: Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), , p. 1665 - 1670 |
|
Biochemical modulation of the catabolism and tissue uptake of the anticancer drug 5-fluorouracil by 5-bromovinyluracil: assessment with metabolic (19)F MR imaging.
Magn. Reson. Med. 42(5) , 936-43, (1999) Using chemical shift-selective (19)F magnetic resonance (MR) imaging, we investigated the biomodulating action of 5-bromovinyluracil (BVU) on the degradation of the anticancer drug 5-fluorouracil (5-F... |
|
|
Deoxyribosyl exchange reactions leading to the in vivo generation and regeneration of the antiviral agents (E)-5-(2-bromovinyl)-2'-deoxyuridine, 5-ethyl-2'-deoxyuridine and 5-(2-chloroethyl)-2'-deoxyuridine.
Biochem. Pharmacol. 35(10) , 1647-53, (1986) In the rat, the highly potent anti-herpes drug (E)-5-(2-bromovinyl)-2'-deoxyuridine (BVdUrd) is rapidly converted to its base (E)-5-(2-bromovinyl)uracil (BVUra) through the action of pyrimidine nucleo... |
|
|
Synthesis and antiviral activity of (E)-5-(2-bromovinyl)uracil and (E)-5-(2-bromovinyl)uridine.
J. Med. Chem. 29 , 213, (1986) (E)-5-(2-Bromovinyl)uracil (BVU) and (E)-5-(2-bromovinyl)uridine (BVRU) were synthesized starting from 5-formyluracil via (E)-5-(2-carboxyvinyl)uracil or starting from 5-iodouridine via (E)-5-(2-carbo... |
| BV uracil |
| 5-bromovinyluracil |
| Bromovinyluracil |
| MFCD00132885 |

 CAS#:66-22-8
CAS#:66-22-8 CAS#:69304-47-8
CAS#:69304-47-8![5-[(E)-2-bromoethenyl]-1-(2-hydroxyethoxymethyl)pyrimidine-2,4-dione structure](https://image.chemsrc.com/caspic/351/91897-93-7.png) CAS#:91897-93-7
CAS#:91897-93-7![FURO[2,3-D]PYRIMIDIN-2(3H)-ONE structure](https://image.chemsrc.com/caspic/457/62785-91-5.png) CAS#:62785-91-5
CAS#:62785-91-5