CRT0044876

CRT0044876 structure
|
Common Name | CRT0044876 | ||
|---|---|---|---|---|
| CAS Number | 6960-45-8 | Molecular Weight | 206.155 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 520.8±30.0 °C at 760 mmHg | |
| Molecular Formula | C9H6N2O4 | Melting Point | 260-261 °C | |
| MSDS | Chinese USA | Flash Point | 268.8±24.6 °C | |
| Symbol |

GHS08 |
Signal Word | Danger | |
Use of CRT0044876CRT0044876 is a potent and selective apurinic/apyrimidinic endonuclease 1 (APE1) inhibitor (IC50=~3 μM). CRT0044876 inhibits the AP endonuclease, 3′-phosphodiesterase and 3′-phosphatase activities of APE1, and is a specific inhibitor of the exonuclease III family of enzymes to which APE1 belongs. CRT0044876 potentiates the cytotoxicity of several DNA base-targeting compounds[1]. |
| Name | 7-Nitroindole-2-carboxylic acid |
|---|---|
| Synonym | More Synonyms |
| Description | CRT0044876 is a potent and selective apurinic/apyrimidinic endonuclease 1 (APE1) inhibitor (IC50=~3 μM). CRT0044876 inhibits the AP endonuclease, 3′-phosphodiesterase and 3′-phosphatase activities of APE1, and is a specific inhibitor of the exonuclease III family of enzymes to which APE1 belongs. CRT0044876 potentiates the cytotoxicity of several DNA base-targeting compounds[1]. |
|---|---|
| Related Catalog | |
| In Vitro | A key step in BER is the processing of an apurinic/apyrimidinic (AP) site intermediate by an AP endonuclease. CRT0044876 has an IC50 for inhibition of APE1 of ∼3 μM and not only inhibits AP site cleavage catalyzed by purified APE1, but also cleavage directed by APE1 in a HeLa whole cell extract. CRT0044876 inhibits the 3′-phosphoglycolate diesterase activity of APE1 with an IC50 of ∼5 μM[1]. CRT0044876 inhibits both the exonuclease and AP endonuclease activities of exonuclease III, but shows no inhibitory activity towards endonuclease IV. CRT0044876 has minimal effects on BamHI restriction endonuclease or topoisomerase I even at CRT0044876 concentrations of 100 μM[1]. At non-toxic concentrations, CRT0044876 potentiates the cytotoxicity of several DNA damaging agents, which generate damage that is repaired in the BER pathway, including some currently-used anticancer drugs. The combination of MMS and CRT0044876 leads to a synergistic increase in the level of AP sites. Consistent with CRT0044876 being a specific BER inhibitor, a strong potentiation of hmdUrd cytotoxicity is seen in CRT0044876-treated cells (HT1080 cells)[1]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 520.8±30.0 °C at 760 mmHg |
| Melting Point | 260-261 °C |
| Molecular Formula | C9H6N2O4 |
| Molecular Weight | 206.155 |
| Flash Point | 268.8±24.6 °C |
| Exact Mass | 206.032761 |
| PSA | 98.91000 |
| LogP | 2.80 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.761 |
| Storage condition | room temp |
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H319-H334 |
| Precautionary Statements | P261-P305 + P351 + P338-P342 + P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R21/22;R36/37/38 |
| Safety Phrases | S36/37/39-S26-S22 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
|
~98% 
CRT0044876 CAS#:6960-45-8 |
| Literature: Purzer Pharmaceutical Co., Ltd. Patent: EP2366687 A2, 2011 ; Location in patent: Page/Page column 8; 10 ; |
|
~96% 
CRT0044876 CAS#:6960-45-8 |
| Literature: LG Life Sciences Ltd. Patent: US2010/210647 A1, 2010 ; Location in patent: Page/Page column 24 ; |
|
~% 
CRT0044876 CAS#:6960-45-8 |
| Literature: Journal of the American Chemical Society, , vol. 80, p. 4621 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
TDP1 deficiency sensitizes human cells to base damage via distinct topoisomerase I and PARP mechanisms with potential applications for cancer therapy.
Nucleic Acids Res. 42(5) , 3089-103, (2014) Base damage and topoisomerase I (Top1)-linked DNA breaks are abundant forms of endogenous DNA breakage, contributing to hereditary ataxia and underlying the cytotoxicity of a wide range of anti-cancer... |
|
|
APE1- and APE2-dependent DNA breaks in immunoglobulin class switch recombination.
J. Exp. Med. 204 , 3017-3026 Antibody class switch recombination (CSR) occurs by an intrachromosomal deletion requiring generation of double-stranded breaks (DSBs) in switch-region DNA. The initial steps in DSB formation have bee... |
|
|
A novel regulatory circuit in base excision repair involving AP endonuclease 1, Creb1 and DNA polymerase beta.
Nucleic Acids Res. 39 , 3156-65, (2011) DNA repair is required to maintain genome stability in stem cells and early embryos. At critical junctures, oxidative damage to DNA requires the base excision repair (BER) pathway. Since early zebrafi... |
| 1H-Indole-2-carboxylic acid, 7-nitro- |
| MFCD00044720 |
| 7-Nitro-1H-indole-2-carboxylic acid |
| EINECS 230-154-6 |
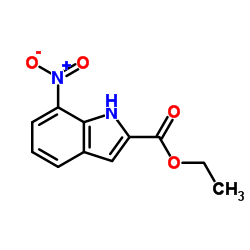
![ethyl 2-[2-(2-nitrophenyl)hydrazinylidene]propanoate structure](https://image.chemsrc.com/caspic/429/292853-66-8.png)
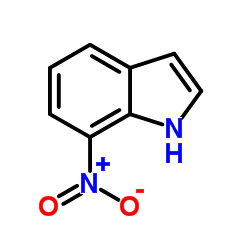 CAS#:6960-42-5
CAS#:6960-42-5 CAS#:5192-04-1
CAS#:5192-04-1
