Cefodizime
Modify Date: 2025-08-20 18:15:36
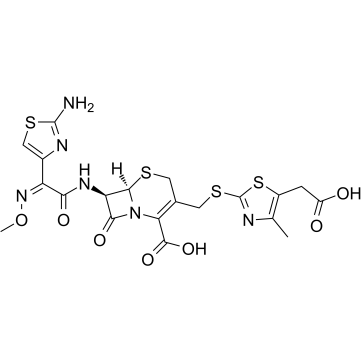
Cefodizime structure
|
Common Name | Cefodizime | ||
|---|---|---|---|---|
| CAS Number | 69739-16-8 | Molecular Weight | 584.669 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C20H20N6O7S4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of CefodizimeCefodizime is a third generation cephalosporin antibiotic with a broad spectrum of antibacterial activity. Cefodizime has no renal toxic effect, good tolerance and immune regulation activity, and is widely used in the treatment of severe infections of the respiratory and urinary tracts[1][2]. |
| Name | cefodizime |
|---|---|
| Synonym | More Synonyms |
| Description | Cefodizime is a third generation cephalosporin antibiotic with a broad spectrum of antibacterial activity. Cefodizime has no renal toxic effect, good tolerance and immune regulation activity, and is widely used in the treatment of severe infections of the respiratory and urinary tracts[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Bacterial[1] |
| In Vitro | Enterobacteriaceae including Escherichia coli, Klebsiella pneumoniae, Morganella morgan ii, Proteus mirabilis, Proteus vulgaris, Shigella sonnei, Yersinia enterocolitica and Salmonella species are all consistently sensitive to Cefodizime in vitro. Cefodizime has marginal but variable inhibitory activity against Citrobacter species including Citrobacter freundii, and Serratia marcescens. Cefodizime inhibits other Gram-negative bacteria including Haemophilus irifluenzae, Moraxella catarrhalis, Neisseria gonorrhoeae and Neisseria meningitidis[1]. Cefodizime is a bactericidal antibiotic having high affinity for penicillin-binding proteins lA/B, 2 and 3 of E. coli. The in vitro concentrations of Cefodizime resulting in bactericidal activity against susceptible strains of Gram-positive and Gram-negative bacteria are generally similar to the minimum inhibitory concentrations[1]. |
| In Vivo | In experimentally-induced K. pneumoniae respiratory tract infections in mice, Cefodizime has activity comparable to Cefotaxime and Ceftazidime, and greater than that of Cefoperazone, Latamoxef, Cefuroxime or cefazolin for 8 hours after a single subcutaneous dose of 50 mg/kg. However, unlike these cephalosporins, Cefodizime continues to demonstrate pronounced bactericidal activity for at least 48 hours after a single injection. Complete bacterial clearance from the lung is achieved within 48 hours in 50% of the mice although Cefodizime is no longer detectable in the serum[1]. |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Molecular Formula | C20H20N6O7S4 |
| Molecular Weight | 584.669 |
| Exact Mass | 584.027649 |
| PSA | 304.48000 |
| LogP | 2.55 |
| Index of Refraction | 1.852 |
| InChIKey | XDZKBRJLTGRPSS-CXAGYDPISA-N |
| SMILES | CON=C(C(=O)NC1C(=O)N2C(C(=O)O)=C(CSc3nc(C)c(CC(=O)O)s3)CSC12)c1csc(N)n1 |
| Storage condition | 2-8°C |
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R36/37/38 |
| Safety Phrases | S22-S26-S36/37/39 |
| WGK Germany | 3 |
|
~%
Detail
|
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 37, # 2 p. 513 - 515 |
| (6R,7R)-7-({(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-[(methyloxy)imino]acetyl}amino)-3-({[5-(carboxymethyl)-4-methyl-1,3-thiazol-2-yl]thio}methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| (6R,7R)-7-{[(2Z)-2-(2-Amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino}-3-({[5-(carboxymethyl)-4-methyl-1,3-thiazol-2-yl]sulfanyl}methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2Z)-2-(2-amino-4-thiazolyl)-2-(methoxyimino)-1-oxoethyl]amino]-3-[[[5-(carboxymethyl)-4-methyl-2-thiazolyl]thio]methyl]-8-oxo-, (6R,7R)- |
| Kenicef |
| MFCD00864926 |
| 7-{[(2-Amino-1,3-thiazol-4-yl)(methoxyimino)acetyl]amino}-3-({[5-(carboxymethyl)-4-methyl-1,3-thiazol-2-yl]sulfanyl}methyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| (6R,7R)-7-[[(2Z)-2-(2-amino-1,3-thiazol-4-yl)-2-methoxyiminoacetyl]amino]-3-[[5-(carboxymethyl)-4-methyl-1,3-thiazol-2-yl]sulfanylmethyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
| [6R-(6a,7b(Z))]-7-[[(2-Amino-4-thiazolyl)(methoxyimino)acetyl]amino]-3-[[[5-(carboxymethyl)-4-methyl-2-thiazolyl]thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid |
| CDZM |
| cefodizimum [INN_la] |
| S 77 1221 B |
| Modivid |
| (6R,7R)-7-[2-(2-Amino-4-thiazolyl)glyoxylamido]-3-[[[5-(carboxymethyl)-4-methyl-2-thiazolyl]thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic Acid 72(Z)-(O-Methyloxime) |
| Neucef |
| Cefodizimum |
| Cefodizima |
| Cefodizme |
| Diezime |
| 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[2-(2-amino-4-thiazolyl)-2-(methoxyimino)acetyl]amino]-3-[[[5-(carboxymethyl)-4-methyl-2-thiazolyl]thio]methyl]-8-oxo- |
| Cefodizime |