adamantanone
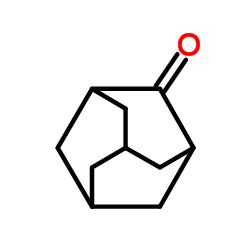
adamantanone structure
|
Common Name | adamantanone | ||
|---|---|---|---|---|
| CAS Number | 700-58-3 | Molecular Weight | 150.218 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 246.7±8.0 °C at 760 mmHg | |
| Molecular Formula | C10H14O | Melting Point | 256-258 °C (subl.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 95.7±10.7 °C | |
Use of adamantanoneAdamantanone (2-Adamantone) is a bioactive chemical, and can be used for the synthesis of active compound[1]. |
| Name | adamantanone |
|---|---|
| Synonym | More Synonyms |
| Description | Adamantanone (2-Adamantone) is a bioactive chemical, and can be used for the synthesis of active compound[1]. |
|---|---|
| Related Catalog |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 246.7±8.0 °C at 760 mmHg |
| Melting Point | 256-258 °C (subl.)(lit.) |
| Molecular Formula | C10H14O |
| Molecular Weight | 150.218 |
| Flash Point | 95.7±10.7 °C |
| Exact Mass | 150.104462 |
| PSA | 17.07000 |
| LogP | 1.60 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.535 |
| InChIKey | IYKFYARMMIESOX-UHFFFAOYSA-N |
| SMILES | O=C1C2CC3CC(C2)CC1C3 |
| Water Solubility | methanol: 0.1 g/mL, clear |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R52 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | AU5018000 |
| HS Code | 29142900 |
| Precursor 9 | |
|---|---|
| DownStream 9 | |
| HS Code | 2914299000 |
|---|---|
| Summary | 2914299000. other cyclanic, cyclenic or cyclotherpenic ketones without other oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
Effect of adamantanone derivative possessing anti-HIV properties on the redox processes in the cytochrome P-450 system.
Dokl. Biochem. Biophys. 378 , 210-3, (2001)
|
|
|
Conformational relaxation in hemoproteins: the cytochrome P-450cam case.
Biochemistry 39(46) , 14219-31, (2000) Photodissociation of (CO)P-450(cam)(substrate) complexes was found to trigger a conformational relaxation process that interferes with ligand rebinding at temperatures as low as 140 K even though the ... |
|
|
The structural basis for substrate-induced changes in redox potential and spin equilibrium in cytochrome P-450CAM.
Biochemistry 28(2) , 917-22, (1989) The crystal structures of cytochrome P-450CAM complexed with the alternative substrates norcamphor and adamantanone have been refined at 2.0-A resolution and compared with the native, camphor-bound fo... |
| 2-Adamantanone |
| MFCD00074737 |
| Tricyclo[3.3.1.1(3,7)]decanone |
| 2-Adamantone |
| 2-Oxoadamantane |
| adamantan-2-one |
| Tricyclo(3,3,1,13,7)decanone |
| Tricyclo[3.3.1.1]decan-2-on |
| EINECS 211-847-2 |
| 4,6-Dehydroadamantanone |
| adamantanone |
| Tricyclo[3.3.1.1]decan-2-one |
 CAS#:700-57-2
CAS#:700-57-2 CAS#:281-23-2
CAS#:281-23-2 CAS#:23695-65-0
CAS#:23695-65-0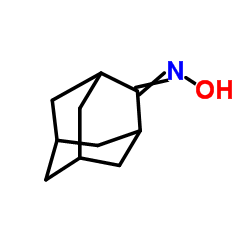 CAS#:4500-12-3
CAS#:4500-12-3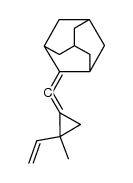 CAS#:118107-74-7
CAS#:118107-74-7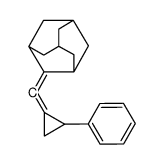 CAS#:99337-53-8
CAS#:99337-53-8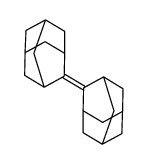 CAS#:30541-56-1
CAS#:30541-56-1 CAS#:113776-99-1
CAS#:113776-99-1 CAS#:201230-82-2
CAS#:201230-82-2![2-[2-(ethylamino)-2-adamantyl]-1-phenylethanol,hydrochloride structure](https://image.chemsrc.com/caspic/018/108736-85-2.png) CAS#:108736-85-2
CAS#:108736-85-2![1-[2-(methylamino)-2-adamantyl]decan-2-ol,hydrochloride structure](https://image.chemsrc.com/caspic/404/108736-88-5.png) CAS#:108736-88-5
CAS#:108736-88-5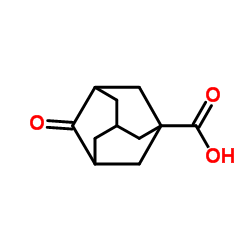 CAS#:56674-87-4
CAS#:56674-87-4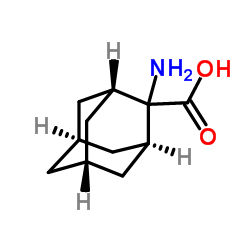 CAS#:42381-05-5
CAS#:42381-05-5 CAS#:491-37-2
CAS#:491-37-2 CAS#:14451-85-5
CAS#:14451-85-5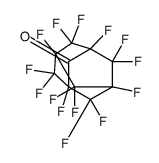 CAS#:141635-73-6
CAS#:141635-73-6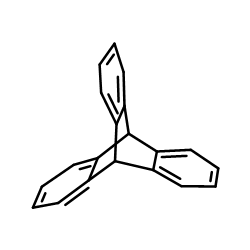 CAS#:477-75-8
CAS#:477-75-8 CAS#:77924-80-2
CAS#:77924-80-2
