CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
WP0700000
-
CHEMICAL NAME :
-
Sulfanilamide, N'-(5-methyl-3-isoxazolyl)-
-
CAS REGISTRY NUMBER :
-
723-46-6
-
BEILSTEIN REFERENCE NO. :
-
0226453
-
LAST UPDATED :
-
199706
-
DATA ITEMS CITED :
-
20
-
MOLECULAR FORMULA :
-
C10-H11-N3-O3-S
-
MOLECULAR WEIGHT :
-
253.30
-
WISWESSER LINE NOTATION :
-
T5NOJ C1 EMSWR DZ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
160 mg/kg/10D-I
-
TOXIC EFFECTS :
-
Endocrine - hypoglycemia
-
REFERENCE :
-
AIMDAP Archives of Internal Medicine. (AMA, 535 N. Dearborn St., Chicago, IL 60610) V.1- 1908- Volume(issue)/page/year: 143,827,1983
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
6200 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 18,185,1971
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
2690 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - convulsions or effect on seizure threshold Lungs, Thorax, or Respiration - dyspnea
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 21,175,1973
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 21,175,1973
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2300 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CMTRAG Chemotherapia. (Basel, Switzerland) V.1-12, 1960-67. For publisher information, see CHTHBK. Volume(issue)/page/year: 8,63,1964
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2300 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - convulsions or effect on seizure threshold Lungs, Thorax, or Respiration - dyspnea
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 21,175,1973
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 21,175,1973
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1460 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
85IRAK "Modern Pharmaceuticals of Japan, V," Tokyo, Japan Pharmaceutical, Medical and Dental Supply Exporters' Assoc., 1975 Volume(issue)/page/year: -,105,1975
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Unreported
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
5 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
CMTRAG Chemotherapia. (Basel, Switzerland) V.1-12, 1960-67. For publisher information, see CHTHBK. Volume(issue)/page/year: 6,273,1963
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
3 gm/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
12ZWAM "Experimental Chemotherapy, Volume II," Schnitzer, R.J., and F. Hawking, eds. Academic Press, New York, 1964 Volume(issue)/page/year: 2,249,1964 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
67500 mg/kg/30D-I
-
TOXIC EFFECTS :
-
Endocrine - changes in thyroid weight Blood - normocytic anemia Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
REFERENCE :
-
NKRZAZ Chemotherapy (Tokyo). (Nippon Kagaku Ryoho Gakkai, 2-20-8 Kamiosaki, Shinagawa-Ku, Tokyo 141, Japan) V.1- 1953- Volume(issue)/page/year: 21,175,1973 ** TUMORIGENIC DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
21 gm/kg/60W-C
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Lungs, Thorax, or Respiration - tumors Endocrine - thyroid tumors
-
REFERENCE :
-
TXAPA9 Toxicology and Applied Pharmacology. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1959- Volume(issue)/page/year: 24,351,1973 *** REVIEWS *** IARC Cancer Review:Animal Limited Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 24,285,1980 IARC Cancer Review:Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 24,285,1980 IARC Cancer Review:Group 3 IMSUDL IARC Monographs, Supplement. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) No.1- 1979- Volume(issue)/page/year: 7,348,1987 TOXICOLOGY REVIEW HFHBAG Hartford Hospital Bulletin. (Hartford Hospital, 80 Seymour St., Hartford, CT 06115) V.1- 1938/39- Volume(issue)/page/year: 29,364,1974 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOHS - National Occupational Hazard Survey (1974) NOHS Hazard Code - 81525 No. of Facilities: 58 (estimated) No. of Industries: 1 No. of Occupations: 2 No. of Employees: 230 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X2070 No. of Facilities: 88 (estimated) No. of Industries: 2 No. of Occupations: 2 No. of Employees: 4250 (estimated) No. of Female Employees: 4016 (estimated) NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - 81525 No. of Facilities: 853 (estimated) No. of Industries: 3 No. of Occupations: 9 No. of Employees: 17028 (estimated) No. of Female Employees: 11965 (estimated)
|
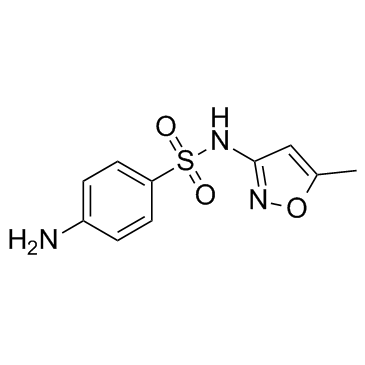


 CAS#:21312-10-7
CAS#:21312-10-7 CAS#:29699-89-6
CAS#:29699-89-6 CAS#:98-74-8
CAS#:98-74-8 CAS#:121-60-8
CAS#:121-60-8 CAS#:114438-33-4
CAS#:114438-33-4 CAS#:1072-67-9
CAS#:1072-67-9 CAS#:6752-38-1
CAS#:6752-38-1 CAS#:13752-78-8
CAS#:13752-78-8 CAS#:141233-20-7
CAS#:141233-20-7![4-[2-(3,5-dioxo-1,2-diphenyl-pyrazolidin-4-ylidene)hydrazinyl]-N-(5-methyloxazol-3-yl)benzenesulfonamide structure](https://image.chemsrc.com/caspic/338/59541-34-3.png) CAS#:59541-34-3
CAS#:59541-34-3
