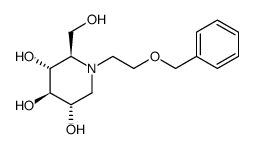
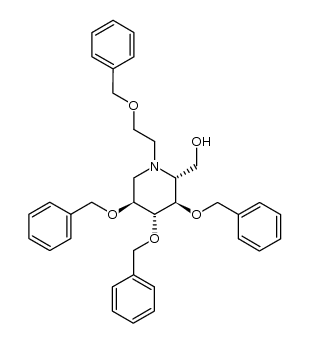
Miglitol

Miglitol structure
|
Common Name | Miglitol | ||
|---|---|---|---|---|
| CAS Number | 72432-03-2 | Molecular Weight | 207.224 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 453.7±45.0 °C at 760 mmHg | |
| Molecular Formula | C8H17NO5 | Melting Point | 114ºC | |
| MSDS | USA | Flash Point | 284.3±27.4 °C | |
Use of MiglitolMiglitol is an oral anti-diabetic drug that acts by inhibiting the ability of the patient to breakdown complex carbohydrates into glucose.Target: OthersMiglitol is an oral anti-diabetic drug that acts by inhibiting the ability of the patient to breakdown complex carbohydrates into glucose. It is primarily used in diabetes mellitus type 2 for establishing greater glycemic control by preventing the digestion of carbohydrates (such as disaccharides, oligosaccharides, and polysaccharides) into monosaccharides which can be absorbed by the body. Miglitol inhibits glycoside hydrolase enzymes called alpha-glucosidases. Since miglitol works by preventing digestion of carbohydrates, it lowers the degree of postprandial hyperglycemia. It must be taken at the start of main meals to have maximal effect. Its effect will depend on the amount of non-monosaccharide carbohydrates in a person's diet. Dietary supplementation with miglitol from pre-onset stage in OLETF rats delays the onset and development of diabetes and preserves the insulin secretory function of pancreatic islets [1]. Miglitol was orally administered at 40 mg/100 g of high-fat diet containing 45% kcal as fat to 12-week-old rats for 29 days, and age-matched rats without the agent were used as the respective controls [2]. |
| Name | Miglitol |
|---|---|
| Synonym | More Synonyms |
| Description | Miglitol is an oral anti-diabetic drug that acts by inhibiting the ability of the patient to breakdown complex carbohydrates into glucose.Target: OthersMiglitol is an oral anti-diabetic drug that acts by inhibiting the ability of the patient to breakdown complex carbohydrates into glucose. It is primarily used in diabetes mellitus type 2 for establishing greater glycemic control by preventing the digestion of carbohydrates (such as disaccharides, oligosaccharides, and polysaccharides) into monosaccharides which can be absorbed by the body. Miglitol inhibits glycoside hydrolase enzymes called alpha-glucosidases. Since miglitol works by preventing digestion of carbohydrates, it lowers the degree of postprandial hyperglycemia. It must be taken at the start of main meals to have maximal effect. Its effect will depend on the amount of non-monosaccharide carbohydrates in a person's diet. Dietary supplementation with miglitol from pre-onset stage in OLETF rats delays the onset and development of diabetes and preserves the insulin secretory function of pancreatic islets [1]. Miglitol was orally administered at 40 mg/100 g of high-fat diet containing 45% kcal as fat to 12-week-old rats for 29 days, and age-matched rats without the agent were used as the respective controls [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 453.7±45.0 °C at 760 mmHg |
| Melting Point | 114ºC |
| Molecular Formula | C8H17NO5 |
| Molecular Weight | 207.224 |
| Flash Point | 284.3±27.4 °C |
| Exact Mass | 207.110672 |
| PSA | 104.39000 |
| LogP | -1.40 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.598 |
| InChIKey | IBAQFPQHRJAVAV-ULAWRXDQSA-N |
| SMILES | OCCN1CC(O)C(O)C(O)C1CO |
| Storage condition | -20°C Freezer |
| Water Solubility | Soluble |
| RIDADR | NONH for all modes of transport |
|---|
|
~95% 
Miglitol CAS#:72432-03-2 |
| Literature: Wennekes, Tom; Meijer, Alfred J.; Groen, Albert K.; Boot, Rolf G.; Groener, Johanna E.; Van Eijk, Marco; Ottenhoff, Roelof; Bijl, Nora; Ghauharali, Karen; Song, Hang; O'Shea, Tom J.; Liu, Hanlan; Yew, Nelson; Copeland, Diane; Van Den Berg, Richard J.; Van Der Marel, Gijsbert A.; Overkleeft, Herman S.; Aerts, Johannes M. Journal of Medicinal Chemistry, 2010 , vol. 53, # 2 p. 689 - 698 |
|
~% 
Miglitol CAS#:72432-03-2 |
| Literature: Tetrahedron Letters, , vol. 52, # 29 p. 3802 - 3804 |
|
~65% 
Miglitol CAS#:72432-03-2 |
| Literature: Concia, Alda Lisa; Lozano, Caries; Castillo, Jose A.; Parella, Teodor; Joglar, Jesus; Clapes, Pere Chemistry - A European Journal, 2009 , vol. 15, # 15 p. 3808 - 3816 |
|
~99% 
Miglitol CAS#:72432-03-2 |
| Literature: Fouace; Therisod Tetrahedron Letters, 2000 , vol. 41, # 38 p. 7313 - 7315 |
|
~% 
Miglitol CAS#:72432-03-2 |
| Literature: Journal of Natural Products, , vol. 65, # 2 p. 198 - 202 |
|
~% 
Miglitol CAS#:72432-03-2 |
| Literature: Tetrahedron Letters, , vol. 52, # 29 p. 3802 - 3804 |
|
~% 
Miglitol CAS#:72432-03-2 |
| Literature: Tetrahedron Letters, , vol. 52, # 29 p. 3802 - 3804 |
|
~% 
Miglitol CAS#:72432-03-2 |
| Literature: Tetrahedron Letters, , vol. 52, # 29 p. 3802 - 3804 |
|
~% 
Miglitol CAS#:72432-03-2 |
| Literature: Tetrahedron Letters, , vol. 41, # 38 p. 7313 - 7315 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
|
Specificity of Processing α-glucosidase I is guided by the substrate conformation: crystallographic and in silico studies.
J. Biol. Chem. 288(19) , 13563-74, (2013) The enzyme “GluI” is key to the synthesis of critical glycoproteins in the cell.We have determined the structure of GluI, and modeled binding with its unique sugar substrate.The specificity of this in... |
|
|
Study of the inhibition of two human maltase-glucoamylases catalytic domains by different α-glucosidase inhibitors.
Carbohydr. Res. 346(17) , 2688-92, (2011) In humans, both the N-terminal catalytic domain (NtMGAM) and the C-terminal catalytic domain (CtMGAM) of small intestinal maltase glucoamylase (MGAM) are α-glycosidases that catalyze the hydrolysis of... |
|
|
Beneficial effects of vildagliptin combined with miglitol on glucose tolerance and islet morphology in diet-controlleddb/dbmice
Biochem. Biophys. Res. Commun. 440(4) , 570-5, (2013) • Beneficial effects of combination therapy with vildagliptin plus miglitol on glucose tolerance of db/db mice were evaluated. • Postprandial glucose- and incretin response was normalized by the combi... |
| BAY m 1099 |
| Miglitol |
| (2R,3R,4R,5S)-1-(2-Hydroxyethyl)-2-(hydroxymethyl)piperidin-3,4,5-triol |
| Diastabol |
| (2R,3R,4R,5S)-1-(2-Hydroxyethyl)-2-(hydroxymethyl)-3,4,5-piperidinetriol |
| EINECS 276-661-6 |
| MFCD00867240 |
| GLYSET |
| Plumarol |
| BAY-m-1099 |
| Seibule |
| (2R,3R,4R,5S)-1-(2-hydroxyethyl)-2-(hydroxymethyl)piperidine-3,4,5-triol |
| N-(2-Hydroxyethyl)moranoline |
| 3,4,5-Piperidinetriol, 1-(2-hydroxyethyl)-2-(hydroxymethyl)-, (2R,3R,4R,5S)- |
| BAY 1099 |
| (2R,3R,4R,5S)-1-(2-hydroxyéthyl)-2-(hydroxyméthyl)pipéridine-3,4,5-triol |