(S)-Benzyl 1-(1H-benzo[d][1,2,3]triazol-1-yl)-1-oxo-3-phenylpropan-2-ylcarbamate
![(S)-Benzyl 1-(1H-benzo[d][1,2,3]triazol-1-yl)-1-oxo-3-phenylpropan-2-ylcarbamate Structure](https://image.chemsrc.com/caspic/275/769922-77-2.png)
(S)-Benzyl 1-(1H-benzo[d][1,2,3]triazol-1-yl)-1-oxo-3-phenylpropan-2-ylcarbamate structure
|
Common Name | (S)-Benzyl 1-(1H-benzo[d][1,2,3]triazol-1-yl)-1-oxo-3-phenylpropan-2-ylcarbamate | ||
|---|---|---|---|---|
| CAS Number | 769922-77-2 | Molecular Weight | 400.43000 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C23H20N4O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of (S)-Benzyl 1-(1H-benzo[d][1,2,3]triazol-1-yl)-1-oxo-3-phenylpropan-2-ylcarbamateZ-Phe-Bt is a phenylalanine derivative[1]. |
| Name | N-Cbz-L-Phe-Bt |
|---|
| Description | Z-Phe-Bt is a phenylalanine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Molecular Formula | C23H20N4O3 |
|---|---|
| Molecular Weight | 400.43000 |
| Exact Mass | 400.15400 |
| PSA | 86.11000 |
| LogP | 4.00010 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| RIDADR | NONH for all modes of transport |
|
Benzotriazole-mediated syntheses of depsipeptides and oligoesters.
J. Org. Chem. 76 , 4884-4893, (2011) Reactions of O-Pg(α-hydroxyacyl)benzotriazoles with (a) unprotected α-hydroxycarboxylic acids, (b) amino acids, and (c) amines afforded, respectively, chirally pure (a) oligoesters, (b) depsidipeptide... |
|
|
Efficient preparation of aminoxyacyl amides, aminoxy hybrid peptides, and alpha-aminoxy peptides.
J. Org. Chem. 74th ed.,, 8690-8694, (2009) N-(Pg-alpha-aminoxy acids) 1a-g are converted to N-(Pg-alpha-aminoxyacyl)benzotriazoles 2a-g, which react under mild conditions with amines, alpha-amino acids/alpha-dipeptides, and alpha-aminoxy acids... |
|
|
Abdelmajeid, A.; Tala, S. R.; Amine, M. S.; Katritzky, A. R.
Synthesis , 2995-3005, (2011)
|
 CAS#:23828-14-0
CAS#:23828-14-0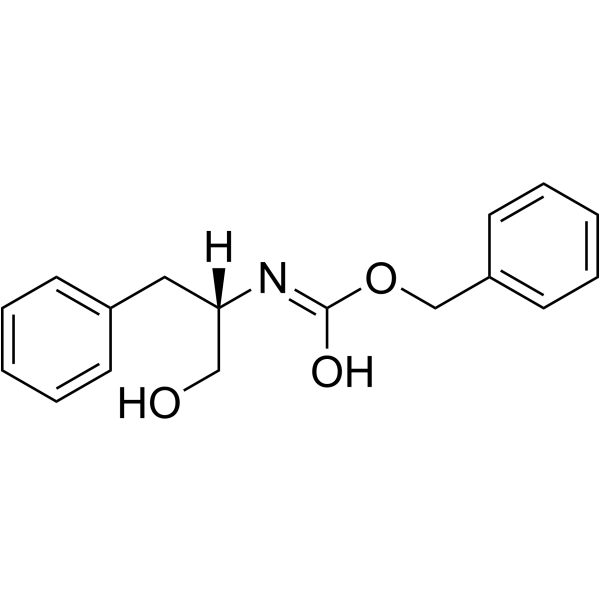 CAS#:6372-14-1
CAS#:6372-14-1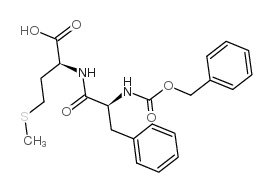 CAS#:13126-07-3
CAS#:13126-07-3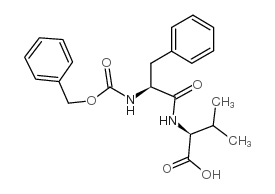 CAS#:13123-00-7
CAS#:13123-00-7 CAS#:13122-91-3
CAS#:13122-91-3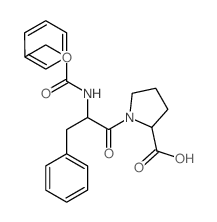 CAS#:7669-64-9
CAS#:7669-64-9 CAS#:37700-64-4
CAS#:37700-64-4