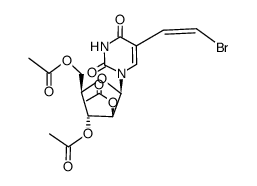
Sorivudine
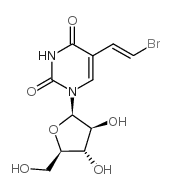
Sorivudine structure
|
Common Name | Sorivudine | ||
|---|---|---|---|---|
| CAS Number | 77181-69-2 | Molecular Weight | 349.13500 | |
| Density | 1.979g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C11H13BrN2O6 | Melting Point | 182 °C | |
| MSDS | N/A | Flash Point | N/A | |
Use of SorivudineSorivudine (BV-araU) is an orally active synthetic pyrimidine nucleoside antimetabolite drug. Sorivudine derives its antiviral activity from selective conversion by a specific thymidine kinase present in certain DNA viruses to nucleotides, which can in turn interfere with viral DNA synthesis[1]. |
| Name | Sorivudine |
|---|---|
| Synonym | More Synonyms |
| Description | Sorivudine (BV-araU) is an orally active synthetic pyrimidine nucleoside antimetabolite drug. Sorivudine derives its antiviral activity from selective conversion by a specific thymidine kinase present in certain DNA viruses to nucleotides, which can in turn interfere with viral DNA synthesis[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Sorivudine (BV-araU) inhibits strains of HSV-1 and HSV-2 (wild-type strains VR-3 and UW-268) with ID50s (50% inhibitory dose) of 0.39 and 0.67 μM, respectively[1]. Sorivudine has antiviral activity against several viruses including varicella zoster virus, herpes simplex type 1 virus, and Epstein-Barr virus[1]. Sorivudine (BV-araU) is a pyrimidine nucleoside analog which has in vitro inhibitory activity against varicella zoster virus (VZV) at concentrations of 00001-0.004 mg/ml These concentrations are over 1000-fold lower than those which are required for the inhibition of VZV replication by acyclovir 3 Sorivudine also inhibits HSV-I replication at concentrations ranging from 0.03-0.1 mg/ml[2]. |
| In Vivo | Sorivudine (BV-araU) has been evaluated in the treatment of HSV-l encephalitis when administered orally to mice. At dosages in excess of 12.5 mg/kg, survival of treated mice is prolonged. With doses in excess of 50 mg/kg, a significant decrease in mortality was achieved as we1l. A more relevant model is that of simian varicella virus infection in African green monkeys (Cerophithecus aethiops). In this system, Sorivudine therapy at dosages as low as 20 mg/kg per day given intramuscularly or 100 mg/kg per day administered orally completely protected against viremia and mortality. In the conduct of these studies, there was no evidence of neurotoxicity or abnormalities in hematology or clinical chemistries. Doses as low as 0.2 mg/kg per day were effective; however, breakthrough viremia was noted at lower dosages[2]. |
| References |
| Density | 1.979g/cm3 |
|---|---|
| Melting Point | 182 °C |
| Molecular Formula | C11H13BrN2O6 |
| Molecular Weight | 349.13500 |
| Exact Mass | 347.99600 |
| PSA | 124.78000 |
| Index of Refraction | 1.746 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
|
~22% 
Sorivudine CAS#:77181-69-2 |
| Literature: RESprotech GmbH Patent: US2010/227834 A1, 2010 ; Location in patent: Page/Page column 20 ; |
|
~% 
Sorivudine CAS#:77181-69-2 |
| Literature: Baraldi, Pier G.; Bazzanini, Rita; Manfredini, Stefano; Simoni, Daniele; Robins, Morris J. Tetrahedron Letters, 1993 , vol. 34, # 19 p. 3177 - 3180 |
|
~% 
Sorivudine CAS#:77181-69-2 |
| Literature: Baraldi, Pier G.; Bazzanini, Rita; Manfredini, Stefano; Simoni, Daniele; Robins, Morris J. Tetrahedron Letters, 1993 , vol. 34, # 19 p. 3177 - 3180 |
|
~% 
Sorivudine CAS#:77181-69-2 |
| Literature: Robins; Manfredini Tetrahedron Letters, 1990 , vol. 31, # 39 p. 5633 - 5636 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| Bravavir |
| Bravavir (tn) |
| Sorivudin |
| YN-72 |
| Brovavir |
| BV-araU |