D149 Dye
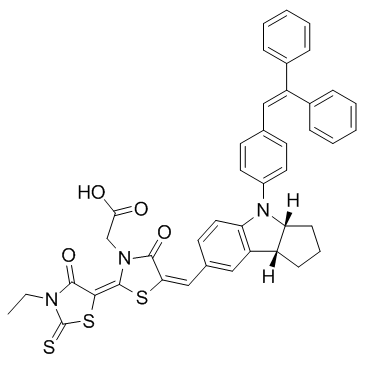
D149 Dye structure
|
Common Name | D149 Dye | ||
|---|---|---|---|---|
| CAS Number | 786643-20-7 | Molecular Weight | 741.94000 | |
| Density | 1.47g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C42H35N3O4S3 | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
Use of D149 DyeD149 Dye is an indoline-based dye, which is a high-extinction-coefficient metal-free organic sensitizer. |
| Name | D-149 Dye |
|---|---|
| Synonym | More Synonyms |
| Description | D149 Dye is an indoline-based dye, which is a high-extinction-coefficient metal-free organic sensitizer. |
|---|---|
| Related Catalog | |
| In Vitro | D149 is a metal-free organic dye, which is promising all-organic alternatives. D149 displays power conversion efficiency of up to 9%. Furthermore, D149 has a peak extinction co-efficient of 68700 M-1 cm-1 at 540 nm, significantly higher than 13900 M-1cm-1 at 535 nm for N719[1]. D149, a metal-free indoline dye, is one of the most promising sensitizers for dye-sensitized solar cells (DSSCs) and has shown very high solar energy conversion efficiencies of 9%. D149 shows a large number of unresolved aromatic and olefinic signals between 7 and 7.5 ppm[2] |
| Cell Assay | The porous TiO2 films are immersed in a 0.5 mM D149 (1-material) dye solution in a 1:1 (v/v) mixture of acetonitrile (HPLC) and tert-butanol (LR) overnight once their temperature decreased to approximately 110°C. The samples are then taken out of the dye bath, washed with acetonitrile, and dried. The working electrode and Pt counter electrode [produced using a pre-drilled piece of 2.3 mm FTO glass, coated with one drop of 10 mM platinic acid solution [H2PtCl6] and heated to 400°C for 20 min] are assembled into a sandwich type cell and sealed with a spacer of 25 μm Surlyn. An I-1/I3-1 organic solvent based electrolyte solution [50 mM iodine, 0.6 M 1,2-dimethyl-3-propylimidazelium iodide, 0.1 M lithium iodide in methoxypropionitrile] is introduced via vacuum back-filling. The hole is sealed with a piece of aluminium foil-backed Surlyn[1]. |
| References |
| Density | 1.47g/cm3 |
|---|---|
| Molecular Formula | C42H35N3O4S3 |
| Molecular Weight | 741.94000 |
| Exact Mass | 741.17900 |
| PSA | 168.48000 |
| LogP | 7.19120 |
| InChIKey | OZFUEQNYOBIXTB-SJIUXOFISA-N |
| SMILES | CCN1C(=O)C(=c2sc(=Cc3ccc4c(c3)C3CCCC3N4c3ccc(C=C(c4ccccc4)c4ccccc4)cc3)c(=O)n2CC(=O)O)SC1=S |
| Storage condition | -20℃ |
| RIDADR | NONH for all modes of transport |
|---|
|
~% 
D149 Dye CAS#:786643-20-7 |
| Literature: Dyes and Pigments, , vol. 91, # 2 p. 145 - 152 |
|
~% 
D149 Dye CAS#:786643-20-7 |
| Literature: Dyes and Pigments, , vol. 91, # 2 p. 145 - 152 |
|
~% 
D149 Dye CAS#:786643-20-7 |
| Literature: Dyes and Pigments, , vol. 91, # 2 p. 145 - 152 |
|
~% 
D149 Dye CAS#:786643-20-7 |
| Literature: Dyes and Pigments, , vol. 91, # 2 p. 145 - 152 |
|
~% 
D149 Dye CAS#:786643-20-7 |
| Literature: Dyes and Pigments, , vol. 91, # 2 p. 145 - 152 |
|
High efficiency of dye-sensitized solar cells based on metal-free indoline dyes.
J. Am. Chem. Soc. 126 , 12218, (2004) We now report metal-free organic dyes having a new type of indoline structure, which exhibits high efficiencies in dye-sensitized solar cells. The solar energy to current conversion efficiencies with ... |
|
|
S. Itoh et. al.
Adv. Mater. 18 , 1202, (2006)
|
| D149 Dye |
![1,2,3,4-Tetrahydrocyclopenta[b]indole structure](https://image.chemsrc.com/caspic/259/2047-91-8.png)
![1,2,3,3a,4,8b-hexahydrocyclopenta[b]indole structure](https://image.chemsrc.com/caspic/440/80278-94-0.png)

![(3aS,8bS)-4-[4-(2,2-diphenylvinyl)phenyl]-2,3,3a,8b-tetrahydro-1H -cyclopenta[b]indole structure](https://image.chemsrc.com/caspic/319/1118752-92-3.png)