potassium t-butoxide
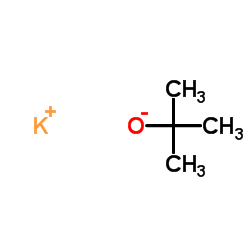
potassium t-butoxide structure
|
Common Name | potassium t-butoxide | ||
|---|---|---|---|---|
| CAS Number | 865-47-4 | Molecular Weight | 112.21 | |
| Density | 0.910 g/mL at 20 °C | Boiling Point | 275°C | |
| Molecular Formula | C4H9KO | Melting Point | 256-258 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 54 °F | |
| Symbol |


GHS02, GHS05 |
Signal Word | Danger | |
Use of potassium t-butoxidePotassium tert-butoxide (Potassiumtbutoxide) is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | Potassium tert-butanolate |
|---|---|
| Synonym | More Synonyms |
| Description | Potassium tert-butoxide (Potassiumtbutoxide) is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 0.910 g/mL at 20 °C |
|---|---|
| Boiling Point | 275°C |
| Melting Point | 256-258 °C (dec.)(lit.) |
| Molecular Formula | C4H9KO |
| Molecular Weight | 112.21 |
| Flash Point | 54 °F |
| Exact Mass | 112.029045 |
| PSA | 23.06000 |
| LogP | 1.21540 |
| Vapour Pressure | 1 mm Hg ( 220 °C) |
| Storage condition | Flammables area |
| Stability | Stable, but reacts violently with water and acids, possibly leading to fire. Incompatible with water, acids, halogenated hydrocarbons, alcohols, strong oxidizing agents, ketones, carbon dioxide. |
| Water Solubility | REACTS |
| Symbol |


GHS02, GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H228-H260-H314 |
| Supplemental HS | Reacts violently with water. |
| Precautionary Statements | P210-P231 + P232-P280-P305 + P351 + P338-P370 + P378-P402 + P404 |
| Personal Protective Equipment | Faceshields;full-face respirator (US);Gloves;Goggles;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | C:Corrosive |
| Risk Phrases | R11;R14;R19;R35 |
| Safety Phrases | S16-S26-S36/37/39-S43-S45-S7/9-S8-S43A-S33-S27 |
| RIDADR | UN 3274 3/PG 2 |
| WGK Germany | 1 |
| Packaging Group | I |
| Hazard Class | 8 |
| HS Code | 29051900 |
|
~% 
potassium t-butoxide CAS#:865-47-4 |
| Literature: US2005/101806 A1, ; Page/Page column 4 ; |
| Precursor 1 | |
|---|---|
| DownStream 10 | |
| HS Code | 2915900090 |
|---|---|
| Summary | 2915900090 other saturated acyclic monocarboxylic acids and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:5.5% General tariff:30.0% |
|
Silylation of C-H bonds in aromatic heterocycles by an Earth-abundant metal catalyst.
Nature 518(7537) , 80-4, (2015) Heteroaromatic compounds containing carbon-silicon (C-Si) bonds are of great interest in the fields of organic electronics and photonics, drug discovery, nuclear medicine and complex molecule synthesi... |
|
|
Encapsulation of fish oil with N-stearoyl O-butylglyceryl chitosan using membrane and ultrasonic emulsification processes.
Carbohydr. Polym. 123 , 432-42, (2015) Fish oil-loaded microcapsules were prepared from oil-in-water emulsions using N-stearoyl O-butylglyceryl chitosan as shell material. The emulsions were prepared by both membrane and ultrasonic emulsif... |
|
|
Utilizing capillary gas chromatography mass spectrometry to determine 4-benzotrifluoride t-butyl ether as a reaction by-product in fluoxetine synthesized using potassium t-butoxide as base.
J. Pharm. Biomed. Anal. 31(1) , 63-74, (2003) Fluoxetine hydrochloride has been prepared using two similar synthetic routes, both of which rely upon an ether formation reaction mediated by a base. The base used can affect the impurity profile of ... |
| potassium,2-methylpropan-2-olate |
| tBuOK |
| Kalium-2-methylpropan-2-olat |
| KOtBu |
| MFCD00012162 |
| potassium t-butoxide |
| Potassium tert-butoxide |
| tert-Butoxide, potassium |
| potassium 2-methylpropan-2-olate |
| EINECS 212-740-3 |
| Potassium 2-methyl-2-propanolate |
| 2-Propanol, 2-methyl-, potassium salt (1:1) |
 CAS#:10602-34-3
CAS#:10602-34-3 CAS#:100-67-4
CAS#:100-67-4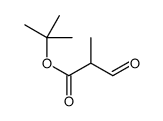 CAS#:110168-50-8
CAS#:110168-50-8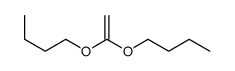 CAS#:10284-51-2
CAS#:10284-51-2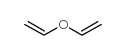 CAS#:109-93-3
CAS#:109-93-3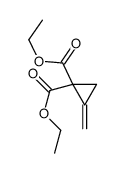 CAS#:106352-19-6
CAS#:106352-19-6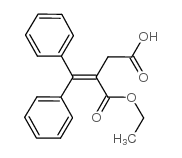 CAS#:5438-22-2
CAS#:5438-22-2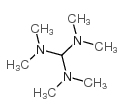 CAS#:5762-56-1
CAS#:5762-56-1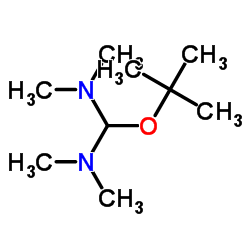 CAS#:5815-08-7
CAS#:5815-08-7 CAS#:36805-97-7
CAS#:36805-97-7
