Methyl salicylate
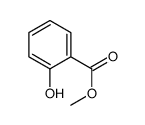
Methyl salicylate structure
|
Common Name | Methyl salicylate | ||
|---|---|---|---|---|
| CAS Number | 90045-28-6 | Molecular Weight | 152.14700 | |
| Density | 1.181 g/mL at 25 °C(lit.) | Boiling Point | N/A | |
| Molecular Formula | C8H8O3 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 96℃ | |
| Symbol |

GHS05, GHS07 |
Signal Word | Danger | |
| Name | Methyl salicylate |
|---|
| Density | 1.181 g/mL at 25 °C(lit.) |
|---|---|
| Molecular Formula | C8H8O3 |
| Molecular Weight | 152.14700 |
| Flash Point | 96℃ |
| Exact Mass | 152.04700 |
| PSA | 46.53000 |
| LogP | 1.17880 |
| Storage condition | 2-8°C |
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H315-H318 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xn |
| Risk Phrases | 22-36/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Quantitative structure-activity relationship and complex network approach to monoamine oxidase A and B inhibitors.
J. Med. Chem. 51 , 6740-51, (2008) The work provides a new model for the prediction of the MAO-A and -B inhibitor activity by the use of combined complex networks and QSAR methodologies. On the basis of the obtained model, we prepared ... |
|
|
Structure-activity relationship of salicylic acid derivatives on inhibition of TNF-α dependent NFκB activity: Implication on anti-inflammatory effect of N-(5-chlorosalicyloyl)phenethylamine against experimental colitis.
Eur. J. Med. Chem. 48 , 36-44, (2012) To develop a more potent NFκB inhibitor from salicylic acid which is known to inhibit activity of NFκB, a transcription factor regulating genes involved in immunity, inflammation and tumorigenesis, de... |