Hemoglobin
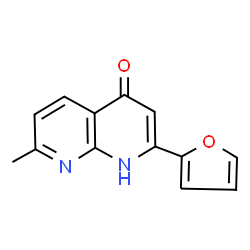
Hemoglobin structure
|
Common Name | Hemoglobin | ||
|---|---|---|---|---|
| CAS Number | 9008-02-0 | Molecular Weight | 226.2307 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 400.9±45.0 °C at 760 mmHg | |
| Molecular Formula | C13H10N2O2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 196.3±28.7 °C | |
Use of HemoglobinHemoglobin is a iron-containing protein in red blood cells with oxygen binding properties. Hemoglobin consits of heme, which binds to oxygen. Hemoglobin also transports other gases, such as carbon dioxide, nitric oxide, hydrogen sulfide and sulfide. Hemoglobin absorbs unneeded oxygen in tissues, as an antioxidant[1]. |
| Name | Hemoglobin |
|---|---|
| Synonym | More Synonyms |
| Description | Hemoglobin is a iron-containing protein in red blood cells with oxygen binding properties. Hemoglobin consits of heme, which binds to oxygen. Hemoglobin also transports other gases, such as carbon dioxide, nitric oxide, hydrogen sulfide and sulfide. Hemoglobin absorbs unneeded oxygen in tissues, as an antioxidant[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 400.9±45.0 °C at 760 mmHg |
| Molecular Formula | C13H10N2O2 |
| Molecular Weight | 226.2307 |
| Flash Point | 196.3±28.7 °C |
| Exact Mass | 226.074234 |
| LogP | 2.95 |
| Appearance of Characters | substrate powder |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.609 |
| InChIKey | INGWEZCOABYORO-UHFFFAOYSA-N |
| SMILES | Cc1ccc2c(=O)cc(-c3ccco3)[nH]c2n1 |
| Storage condition | 2-8°C |
| Water Solubility | 0.6 M HCl: soluble20mg/mL | Soluble in water. |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | B |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
In vitro digestibility of goat milk and kefir with a new standardised static digestion method (INFOGEST cost action) and bioactivities of the resultant peptides.
Food Funct. 6 , 2322-30, (2015) The hydrolysis degrees of goat milk and kefir during simulated gastrointestinal digestion and some bioactivities of the resulting peptides after fermentation and digestion were studied. A static in vi... |
|
|
Extensive Charge Reduction and Dissociation of Intact Protein Complexes Following Electron Transfer on a Quadrupole-Ion Mobility-Time-of-Flight MS.
J. Am. Soc. Mass Spectrom. 26 , 1068-76, (2015) Non-dissociative charge reduction, typically considered to be an unwanted side reaction in electron transfer dissociation (ETD) experiments, can be enhanced significantly in order to reduce the charge... |
|
|
Effects of sodium dodecyl sulphate on enhancement of lipoxygenase activity of hemoglobin.
Indian J. Exp. Biol. 50(12) , 847-52, (2012) Lipoxygenases comprise a family of non-heme iron-containing enzymes that catalyze the stereospecific dioxygenation of polyunsaturated fatty acids with 1, 4-cis-cis-pentadiene structure. Hemoglobin, a ... |
| MFCD00131282 |
| Deoxyhemoglobins |
| Ferrohemoglobins |
| Hemoglobin human |
| Blood pigments |

