(R)-Alcohol dehydrogenase
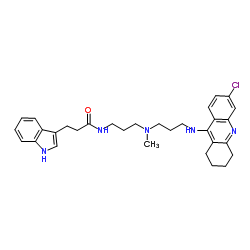
(R)-Alcohol dehydrogenase structure
|
Common Name | (R)-Alcohol dehydrogenase | ||
|---|---|---|---|---|
| CAS Number | 9028-12-0 | Molecular Weight | 532.119 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 806.8±65.0 °C at 760 mmHg | |
| Molecular Formula | C31H38ClN5O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 441.7±34.3 °C | |
| Symbol |

GHS08 |
Signal Word | Danger | |
Use of (R)-Alcohol dehydrogenase(R)-Alcohol dehydrogenase (E.C. 1.1.1.2) is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | Dehydrogenase, Alcohol(Nicotinamide Adenine Dinucleotide Phosphate) |
|---|---|
| Synonym | More Synonyms |
| Description | (R)-Alcohol dehydrogenase (E.C. 1.1.1.2) is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 806.8±65.0 °C at 760 mmHg |
| Molecular Formula | C31H38ClN5O |
| Molecular Weight | 532.119 |
| Flash Point | 441.7±34.3 °C |
| Exact Mass | 531.276489 |
| LogP | 5.89 |
| Vapour Pressure | 0.0±2.9 mmHg at 25°C |
| Index of Refraction | 1.663 |
| Symbol |

GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H317-H334 |
| Precautionary Statements | P261-P280-P342 + P311 |
| Personal Protective Equipment | Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | 42/43 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
Efficient one-step production of (S)-1-phenyl-1,2-ethanediol from (R)-enantiomer plus NAD(+)-NADPH in-situ regeneration using engineered Escherichia coli.
Microb. Cell Fact. 11 , 167, (2012) Candida parapsilosis CCTCC M203011 catalyzes the stereoinversion of (R)-1-phenyl-1,2-ethanediol (PED) through oxidation and reduction. Its NAD(+)-linked (R)-carbonyl reductase (RCR) catalyzes the oxid... |
|
|
A new strategy to improve the efficiency and sustainability of Candida parapsilosis catalyzing deracemization of (R,S)-1-phenyl-1,2-ethanediol under non-growing conditions: increase of NADPH availability.
J. Microbiol. Biotechnol. 19(1) , 65-71, (2009) Microbial oxidoreductive systems have been widely used in asymmetric syntheses of optically active alcohols. However, when reused in multi-batch reaction, the catalytic efficiency and sustainability o... |
|
|
Stereospecificity of ketoreductase domains 1 and 2 of the tylactone modular polyketide synthase.
J. Am. Chem. Soc. 130 , 11598-11599, (2008) Tylactone synthase (TYLS) is a modular polyketide synthase that catalyzes the formation of tylactone (1), the parent aglycone precursor of the macrolide antibiotic tylosin. TYLS modules 1 and 2 are re... |
| 1H-Indole-3-propanamide, N-[3-[[3-[(6-chloro-1,2,3,4-tetrahydro-9-acridinyl)amino]propyl]methylamino]propyl]- |
| N-{3-[{3-[(6-Chloro-1,2,3,4-tetrahydro-9-acridinyl)amino]propyl}(methyl)amino]propyl}-3-(1H-indol-3-yl)propanamide |
| N-{3-[{3-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]propyl}(methyl)amino]propyl}-3-(1H-indol-3-yl)propanamide |