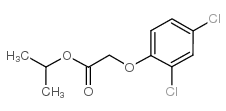
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
AG8750000
-
CHEMICAL NAME :
-
Acetic acid, (2,4-dichlorophenoxy)-, isopropyl ester
-
CAS REGISTRY NUMBER :
-
94-11-1
-
BEILSTEIN REFERENCE NO. :
-
2333761
-
LAST UPDATED :
-
199612
-
DATA ITEMS CITED :
-
11
-
MOLECULAR FORMULA :
-
C11-H12-Cl2-O3
-
MOLECULAR WEIGHT :
-
263.13
-
WISWESSER LINE NOTATION :
-
GR CG DO1VOY1&1
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
700 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
FMCHA2 Farm Chemicals Handbook. (Meister Pub., 37841 Euclid Ave., Willoughy, OH 44094) Volume(issue)/page/year: -,C174,1991
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
541 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - gastritis Behavioral - somnolence (general depressed activity) Liver - fatty liver degeneration
-
REFERENCE :
-
AJVRAH American Journal of Veterinary Research. (American Veterinary Medical Assoc., 930 N. Meacham Rd., Schaumburg, IL 60196) V.1- 1940- Volume(issue)/page/year: 15,622,1954
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - guinea pig
-
DOSE/DURATION :
-
550 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - gastritis Behavioral - somnolence (general depressed activity) Liver - fatty liver degeneration
-
REFERENCE :
-
AJVRAH American Journal of Veterinary Research. (American Veterinary Medical Assoc., 930 N. Meacham Rd., Schaumburg, IL 60196) V.1- 1940- Volume(issue)/page/year: 15,622,1954
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Bird - chicken
-
DOSE/DURATION :
-
1420 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - gastritis Behavioral - somnolence (general depressed activity) Liver - fatty liver degeneration
-
REFERENCE :
-
AJVRAH American Journal of Veterinary Research. (American Veterinary Medical Assoc., 930 N. Meacham Rd., Schaumburg, IL 60196) V.1- 1940- Volume(issue)/page/year: 15,622,1954 ** TUMORIGENIC DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
12 gm/kg/78W-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Lungs, Thorax, or Respiration - tumors Blood - tumors
-
REFERENCE :
-
NTIS** National Technical Information Service. (Springfield, VA 22161) Formerly U.S. Clearinghouse for Scientific & Technical Information. Volume(issue)/page/year: PB223-159 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1326 mg/kg
-
SEX/DURATION :
-
female 7-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
AECTCV Archives of Environmental Contamination and Toxicology. (Springer-Verlag New York, Inc., Service Center, 44 Hartz Way, Secaucus, NJ 070944) V.1- 1973- Volume(issue)/page/year: 6,33,1977
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
846 mg/kg
-
SEX/DURATION :
-
female 6-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - extra-embryonic structures (e.g., placenta, umbilical cord) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - eye/ear
-
REFERENCE :
-
NTIS** National Technical Information Service. (Springfield, VA 22161) Formerly U.S. Clearinghouse for Scientific & Technical Information. Volume(issue)/page/year: PB223-160
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
846 mg/kg
-
SEX/DURATION :
-
female 6-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
REFERENCE :
-
NTIS** National Technical Information Service. (Springfield, VA 22161) Formerly U.S. Clearinghouse for Scientific & Technical Information. Volume(issue)/page/year: PB223-160
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
414 mg/kg
-
SEX/DURATION :
-
female 6-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
REFERENCE :
-
NTIS** National Technical Information Service. (Springfield, VA 22161) Formerly U.S. Clearinghouse for Scientific & Technical Information. Volume(issue)/page/year: PB223-160 *** REVIEWS *** IARC Cancer Review:Animal Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man. (WHO Publications Centre USA, 49 Sheridan Ave., Albany, NY 12210) V.1- 1972- Volume(issue)/page/year: 15,111,1977 *** U.S. STANDARDS AND REGULATIONS *** EPA FIFRA 1988 PESTICIDE SUBJECT TO REGISTRATION OR RE-REGISTRATION FEREAC Federal Register. (U.S. Government Printing Office, Supt. of Documents, Washington, DC 20402) V.1- 1936- Volume(issue)/page/year: 54,7740,1989
|