Cyproconazole
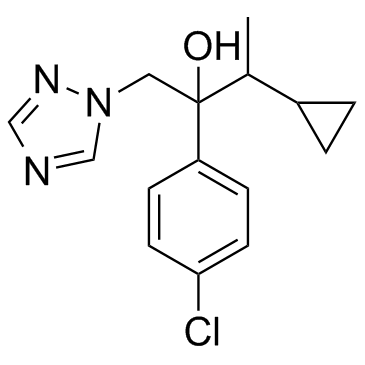
Cyproconazole structure
|
Common Name | Cyproconazole | ||
|---|---|---|---|---|
| CAS Number | 94361-06-5 | Molecular Weight | 291.77600 | |
| Density | 1.32 | Boiling Point | >250ºC | |
| Molecular Formula | C15H18ClN3O | Melting Point | 106.2-106.9ºC | |
| MSDS | Chinese USA | Flash Point | >100 °C | |
| Symbol |



GHS07, GHS08, GHS09 |
Signal Word | Warning | |
Use of CyproconazoleCyproconazole is a triazole fungicide that is used agriculturally for protection of crops against a wide variety of fungal pathogens.In vitro: Cyproconazole has been shown to cause a dose dependent inhibition of progesterone production in human placental cells in vitro. cyproconazole exhibited the lowest capacity to increase CYP1A1 and were not able to activate the AhR in the transactivation assay. [1]In vivo: Cyproconazole, a triazole fungicide, causes hepatocellular adenomas and carcinomas in CD-1 mice at dose levels of 100 and 200 ppm. In wild-type mice, 200 ppm cyproconazole caused liver hypertrophy, increased liver weight and cell proliferation, single-cell necrosis and fat vacuolation. [2] |
| Name | cyproconazole |
|---|---|
| Synonym | More Synonyms |
| Description | Cyproconazole is a triazole fungicide that is used agriculturally for protection of crops against a wide variety of fungal pathogens.In vitro: Cyproconazole has been shown to cause a dose dependent inhibition of progesterone production in human placental cells in vitro. cyproconazole exhibited the lowest capacity to increase CYP1A1 and were not able to activate the AhR in the transactivation assay. [1]In vivo: Cyproconazole, a triazole fungicide, causes hepatocellular adenomas and carcinomas in CD-1 mice at dose levels of 100 and 200 ppm. In wild-type mice, 200 ppm cyproconazole caused liver hypertrophy, increased liver weight and cell proliferation, single-cell necrosis and fat vacuolation. [2] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.32 |
|---|---|
| Boiling Point | >250ºC |
| Melting Point | 106.2-106.9ºC |
| Molecular Formula | C15H18ClN3O |
| Molecular Weight | 291.77600 |
| Flash Point | >100 °C |
| Exact Mass | 291.11400 |
| PSA | 50.94000 |
| LogP | 2.86540 |
| InChIKey | UFNOUKDBUJZYDE-UHFFFAOYSA-N |
| SMILES | CC(C1CC1)C(O)(Cn1cncn1)c1ccc(Cl)cc1 |
| Storage condition | 2-8C |
| Symbol |



GHS07, GHS08, GHS09 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H361-H410 |
| Precautionary Statements | P273-P281-P501 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn |
| Risk Phrases | R22 |
| Safety Phrases | S36/37-S60-S61 |
| RIDADR | UN3077 9/PG 3 |
| RTECS | XZ4803250 |
| Hazard Class | 9.0 |
| HS Code | 2933990015 |
| HS Code | 2933990015 |
|---|---|
| Summary | 2933990015 3-((1h-1,2,4-triazol-1-yl)methyl)-1-(4-chlorophenyl)-4,4-dimethylpentan-3-ol。supervision conditions:s(import or export registration certificate for pesticides)。VAT:17.0%。tax rebate rate:9.0%。MFN tarrif:6.5%。general tariff:20.0% |
|
Is the amphibian X. laevis WEC a good alternative method to rodent WEC teratogenicity assay? The example of the three triazole derivative fungicides Triadimefon, Tebuconazole, Cyproconazole.
Reprod. Toxicol. 32(2) , 220-6, (2011) The aim of the present work is the assessment of teratogenic effects of three triazole-derived fungicides (Triadimefon, FON, Tebuconazole, TEBU, Cyproconazole, CYPRO) on rat and Xenopus laevis embryos... |
|
|
Dose-response involvement of constitutive androstane receptor in mouse liver hypertrophy induced by triazole fungicides.
Toxicol. Lett. 221(1) , 47-56, (2013) To clarify the dose-response relationship between constitutive androstane receptor (CAR) activity and induction of cytochrome P450 2B (CYP2B) expression and hypertrophy by triazole fungicides in mouse... |
|
|
[Behaviour azole fungicide and fluconazole in Cryptococcus neoformans clinical and environmental isolates].
Rev. Soc. Bras. Med. Trop. 40(2) , 209-11, (2007) The activity of azole fungicides for agronomical use (epoxiconazole, difenoconazole and cyproconazole) was evaluated in comparison with the therapeutic antifungal agent fluconazole, on 23 environmenta... |
| 1H-1,2,4-Triazole-1-ethanol,a-(4-chlorophenyl)-a-(1-cyclopropylethyl)- |
| Cyproconazol |
| 2RS,3SR)-2-(4-chlorophenyl)-3-cyclopropyl-1-(1H-1,2,4-triazol-1-yl)butan-2-ol |
| 2-(4-CHLOROPHENYL)-3-CYCLOPROPYL-1-(1,2,4-TRIAZOL-1-YL)BUTAN-2-OL |
| 2-(4-Chlorophenyl)-3-cyclopropyl-1-(1H-1,2,4-triazol-1-yl)butan-2-ol |
| rac-(2R,3RS)-2-(4-chlorophenyl)-3-cyclopropyl-1-(1H-1,2,4-triazol-1-yl)butan-2-ol |
| (2RS,3RS |
| α-(4-chlorophenyl)-α-(1-cyclopropylethyl)-1H-1,2,4-triazole-1-ethanol |
| MFCD01678672 |