Beclabuvir
Modify Date: 2024-01-08 10:33:48
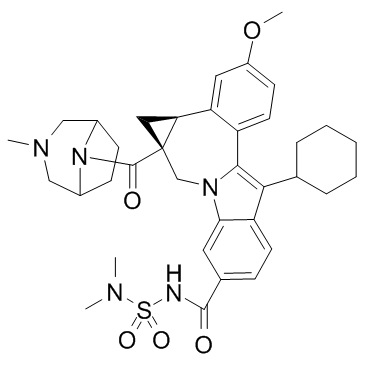
Beclabuvir structure
|
Common Name | Beclabuvir | ||
|---|---|---|---|---|
| CAS Number | 958002-33-0 | Molecular Weight | 659.838 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C36H45N5O5S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of BeclabuvirBeclabuvir is an allosteric inhibitor that binds to thumb site 1 of the hepatitis C virus (HCV) NS5B RNA-dependent RNA polymerase, and inhibits recombinant NS5B proteins from HCV genotypes 1, 3, 4, and 5 with IC50 of < 28 nM. |
| Name | Beclabuvir |
|---|---|
| Synonym | More Synonyms |
| Description | Beclabuvir is an allosteric inhibitor that binds to thumb site 1 of the hepatitis C virus (HCV) NS5B RNA-dependent RNA polymerase, and inhibits recombinant NS5B proteins from HCV genotypes 1, 3, 4, and 5 with IC50 of < 28 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: < 28 nM (NS5B protein) |
| In Vitro | Beclabuvir demonstrates additive or synergistic antiviral activity with pegIFN/RBV and in 2- or 3-drug combinations with a range of DAAs, such as HCV NS3 protease inhibitors, NS5A inhibitors' and/or nucleoside NS5B inhibitors[2]. |
| In Vivo | The combination of beclabuvir with asunaprevir and daclatasvir achieves very high rates of viral eradication (about 90%) in patients infected with HCV genotype 1, which is the most common genotype worldwide[1]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Molecular Formula | C36H45N5O5S |
| Molecular Weight | 659.838 |
| Exact Mass | 659.314148 |
| LogP | 4.71 |
| Index of Refraction | 1.722 |
| Storage condition | 2-8℃ |
| Beclabuvir |
| (1aR,12bS)-8-Cyclohexyl-N-(dimethylsulfamoyl)-11-methoxy-1a-{[(1R,5S)-3-methyl-3,8-diazabicyclo[3.2.1]oct-8-yl]carbonyl}-1,1a,2,12b-tetrahydrocyclopropa[d]indolo[2,1-a][2]benzazepine-5-carboxamide |
| Cycloprop[d]indolo[2,1-a][2]benzazepine-5-carboxamide, 8-cyclohexyl-N-[(dimethylamino)sulfonyl]-1,1a,2,12b-tetrahydro-11-methoxy-1a-[[(1R,5S)-3-methyl-3,8-diazabicyclo[3.2.1]oct-8-yl]carbonyl]-, (1aR,12bS)- |
| MYW1X5CO9S |
| BMS-791325 |