Fc 11a-2
Modify Date: 2025-08-27 19:12:19
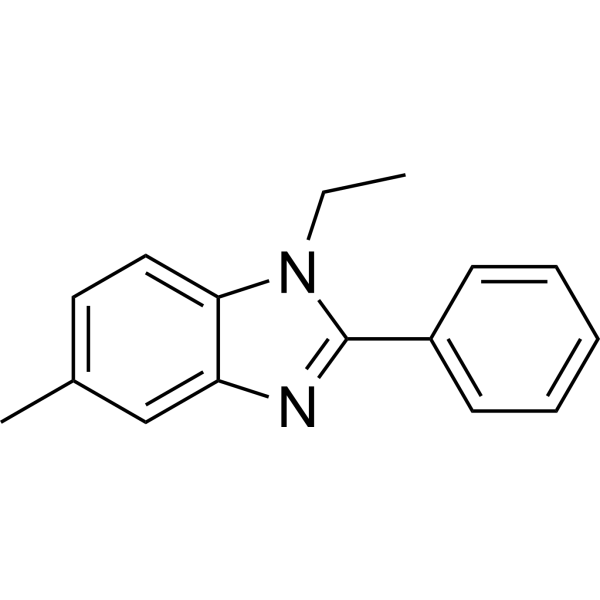
Fc 11a-2 structure
|
Common Name | Fc 11a-2 | ||
|---|---|---|---|---|
| CAS Number | 960119-75-9 | Molecular Weight | 236.31 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C16H16N2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Fc 11a-2Fc 11a-2, a benzimidazole compound, is an orally active and potent NLRP3 inflammasome inhibitor. Fc 11a-2 restrains the formation of NLRP3 inflammasome by inhibiting activation of caspase-1 and thus the activation of IL-1b/IL-18. Fc 11a-2 prevents the development of Dextran sulfate sodium (DSS; HY-116282C)-induced murine experimental colitis[1][2][3]. |
| Name | Fc 11a-2 |
|---|
| Description | Fc 11a-2, a benzimidazole compound, is an orally active and potent NLRP3 inflammasome inhibitor. Fc 11a-2 restrains the formation of NLRP3 inflammasome by inhibiting activation of caspase-1 and thus the activation of IL-1b/IL-18. Fc 11a-2 prevents the development of Dextran sulfate sodium (DSS; HY-116282C)-induced murine experimental colitis[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Fc 11a-2 (10 μM) 抑制用 ATP 激活的 LPS (HY-D1056) 引发的 THP-1 细胞中释放 IL-1β 和 IL-18,IC50 约 10 μM[1]。 |
| In Vivo | Fc 11a-2 (3-30 mg/kg; 口服; 从第 1 天到第 10 天) 剂量依赖性地减轻由 DSS 诱导的体重减轻和结肠长度缩短[2]。 Animal Model: C57BL/6 mice induced Colitis with 2.5% DSS[2] Dosage: 3, 10, 30 mg/kg Administration: Orally; from day 1 to day 10 Result: Significantly attenuated the loss of body weight during the disease progression at 10 and 30 mg/kg. The myeloperoxidase (MPO) activity in colons at 10 and 30 mg/kg was lower than that of the vehicle-treated group. Significantly reduced the disease activity index, histopathologic scores and myeloperoxidase activity. |
| References |
| Molecular Formula | C16H16N2 |
|---|---|
| Molecular Weight | 236.31 |
| InChIKey | LAPXJMHYUYXOQN-UHFFFAOYSA-N |
| SMILES | CCn1c(-c2ccccc2)nc2cc(C)ccc21 |