Prunasin
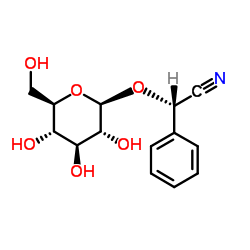
Prunasin structure
|
Common Name | Prunasin | ||
|---|---|---|---|---|
| CAS Number | 99-18-3 | Molecular Weight | 295.29 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 527.0±50.0 °C at 760 mmHg | |
| Molecular Formula | C14H17NO6 | Melting Point | 138-148ºC | |
| MSDS | Chinese USA | Flash Point | 272.5±30.1 °C | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
Use of PrunasinPrunasin is a inhibitor of DNA Polymerase β[1]. |
| Name | (R)-prunasin |
|---|---|
| Synonym | More Synonyms |
| Description | Prunasin is a inhibitor of DNA Polymerase β[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 527.0±50.0 °C at 760 mmHg |
| Melting Point | 138-148ºC |
| Molecular Formula | C14H17NO6 |
| Molecular Weight | 295.29 |
| Flash Point | 272.5±30.1 °C |
| Exact Mass | 295.105591 |
| PSA | 123.17000 |
| LogP | -0.92 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.621 |
| InChIKey | ZKSZEJFBGODIJW-GMDXDWKASA-N |
| SMILES | N#CC(OC1OC(CO)C(O)C(O)C1O)c1ccccc1 |
| Storage condition | Hygroscopic, -20°C Freezer, Under Inert Atmosphere |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H360 |
| Precautionary Statements | P201-P301 + P310-P308 + P313 |
| Hazard Codes | T |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26-36/37/39 |
| RIDADR | UN 2811 6.1 / PGIII |
| RTECS | UL3420000 |
|
~73% 
Prunasin CAS#:99-18-3 |
| Literature: Nakajima, Noriyuki; Ubukata, Makoto Bioscience, Biotechnology and Biochemistry, 1998 , vol. 62, # 3 p. 453 - 458 |
|
~% 
Prunasin CAS#:99-18-3 |
| Literature: Bioscience, Biotechnology and Biochemistry, , vol. 62, # 3 p. 453 - 458 |
|
~% 
Prunasin CAS#:99-18-3 |
| Literature: Bioscience, Biotechnology and Biochemistry, , vol. 62, # 3 p. 453 - 458 |
|
~% 
Prunasin CAS#:99-18-3 |
| Literature: Bioscience, Biotechnology and Biochemistry, , vol. 62, # 3 p. 453 - 458 |
|
~% 
Prunasin CAS#:99-18-3 |
| Literature: Chemical and Pharmaceutical Bulletin, , vol. 57, # 2 p. 207 - 210 |
|
Chromatographic determination of cyanoglycosides prunasin and amygdalin in plant extracts using a porous graphitic carbon column.
J. Agric. Food Chem. 50(24) , 6960-3, (2002) The determination of cyanogenic compounds in plants is often performed by HPLC. However, in this analysis, interferences due to compounds in the matrix, such as tannins and other pigments, are encount... |
|
|
Larvae of the fall webworm, Hyphantria cunea, inhibit cyanogenesis in Prunus serotina.
J. Immunol. Methods 211 , 671-7, (2008) The larvae of the fall webworm, Hyphantria cunea (Dru.), though vulnerable to cyanide poisoning, consume the cyanogenic leaves of black cherry, Prunus serotina, without apparent harm. The cyanide cont... |
|
|
Generation of primary amide glucosides from cyanogenic glucosides
Phytochemistry 70(3) , 388-93, (2009) The conversion of the cyanogenic glucoside prunasin into the corresponding prunasinamide has been observed in the leaves of Olinia ventosa and other prunasin-containing species only if reactive oxygen... |
| Prunasin |
| (2S)-(β-D-Glucopyranosyloxy)(phenyl)acetonitrile |
| (2R)-2-phenyl-2-[(2R,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyacetonitrile |
| D-Prunasin |
| Benzeneacetonitrile, α-(β-D-glucopyranosyloxy)-, (αS)- |
![(αR)-α-[(2,3,4,6-Tetra-O-acetyl-β-D-glucopyranosyl)oxy]benzeneacetamide structure](https://image.chemsrc.com/caspic/084/207512-68-3.png)

 CAS#:100-52-7
CAS#:100-52-7 CAS#:611-71-2
CAS#:611-71-2
