| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
吡罗昔康
CAS:36322-90-4 |
|
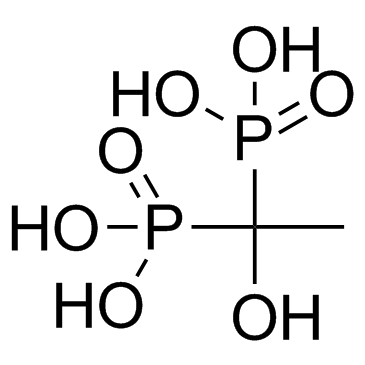 |
羟基乙叉二膦酸(HEDP)
CAS:2809-21-4 |
|
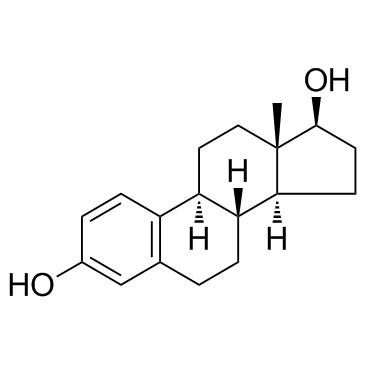 |
雌二醇
CAS:50-28-2 |
|
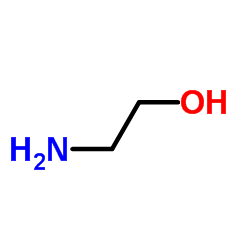 |
2-氨基乙醇
CAS:141-43-5 |
|
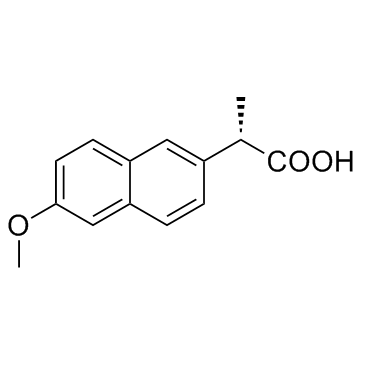 |
萘普生
CAS:22204-53-1 |
|
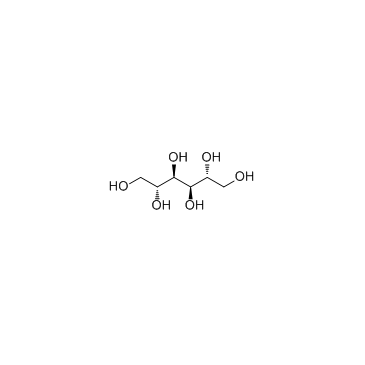 |
甘露醇
CAS:69-65-8 |
|
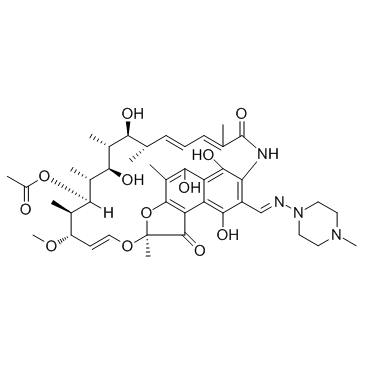 |
利福平
CAS:13292-46-1 |
|
 |
沙丁胺醇
CAS:18559-94-9 |
|
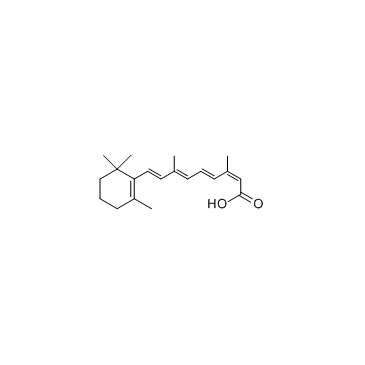 |
异维A酸
CAS:4759-48-2 |
|
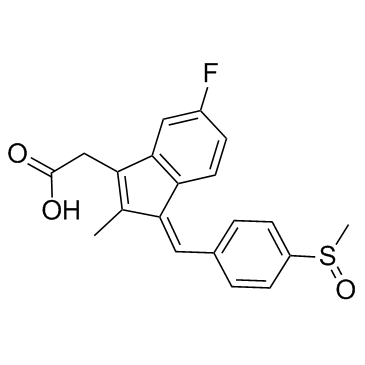 |
舒林酸
CAS:38194-50-2 |