| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Piroxicam
CAS:36322-90-4 |
|
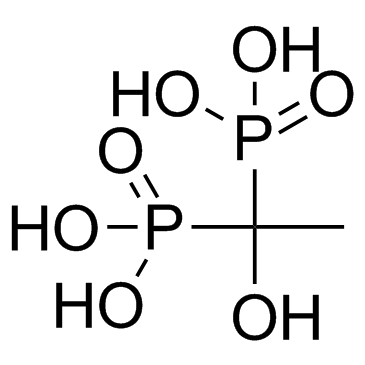 |
Etidronic acid
CAS:2809-21-4 |
|
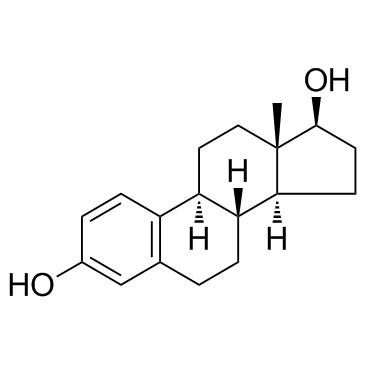 |
beta-Estradiol
CAS:50-28-2 |
|
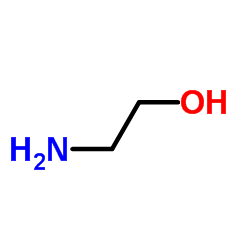 |
2-Aminoethanol
CAS:141-43-5 |
|
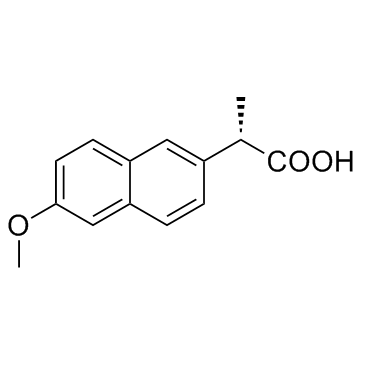 |
Naproxen
CAS:22204-53-1 |
|
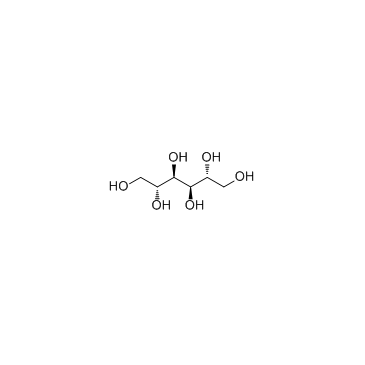 |
D-Mannitol
CAS:69-65-8 |
|
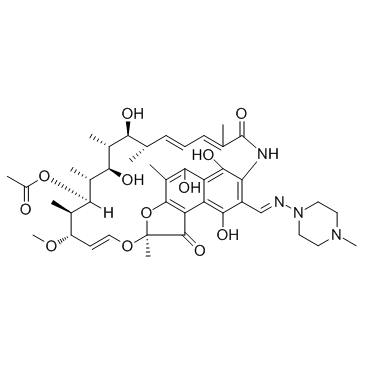 |
Rifampicin
CAS:13292-46-1 |
|
 |
Salbutamol
CAS:18559-94-9 |
|
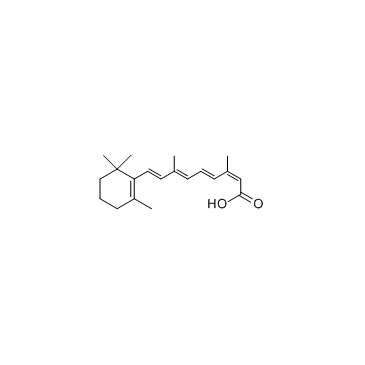 |
Isotretinoin
CAS:4759-48-2 |
|
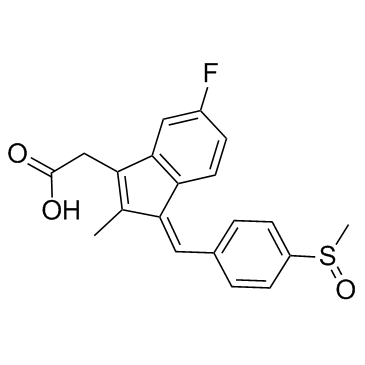 |
Sulindac
CAS:38194-50-2 |