| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
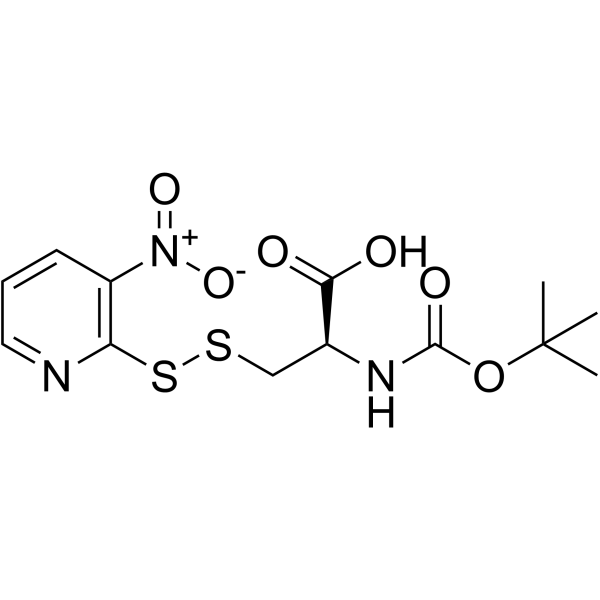 |
(R)-2-((叔丁氧基羰基)氨基)-3-((3-硝基吡啶-2-基)二硫烷基)丙酸
CAS:76880-29-0 |
| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
(R)-2-((叔丁氧基羰基)氨基)-3-((3-硝基吡啶-2-基)二硫烷基)丙酸
CAS:76880-29-0 |