| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
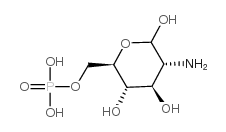 |
D-氨基葡萄糖6-磷酸
CAS:3616-42-0 |
|
 |
D-葡萄糖胺6-磷酸 钠盐
CAS:70442-23-8 |